Who is considered the father of the Periodic Table
Dmitri Mendeleev
The simplest form of matter is the
Atom
She discovered Radium with her husband and went on to win 2(!) Nobel Prizes
Marie Curie
Define the following terms:
Fission
Fusion
fission - Splitting of a nucleus
Fusion - joining of 2 nuclei
What is an Ionic Bond?
A bond where electrons are completely transferred between a metal and a nonmetal.
Reactions that release energy, rather than absorb.
Exothermic
2 Properties that increases as we go up a group.
Electronegativity, Ionization energy
What is happening at each flat line.
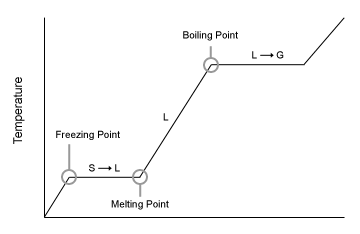
Freezing/melting
Boiling/condensing
John Dalton came up with the Atomic Theory back in 1808. We still follow almost all of these postulates today. Name 3 postulates (bullet points) from this theory.
- All matter consists of indivisible particles called atoms.
- Atoms of the same element are similar in physical and chemical properties.
- Atoms of different elements differ in physical and chemical properties.
- Atoms of different elements combine with each other in a fixed, simple, whole number ratios to form compound atoms.
- In chemical reactions, atoms are combined, separated, or rearranged, but can never be created or destroyed.
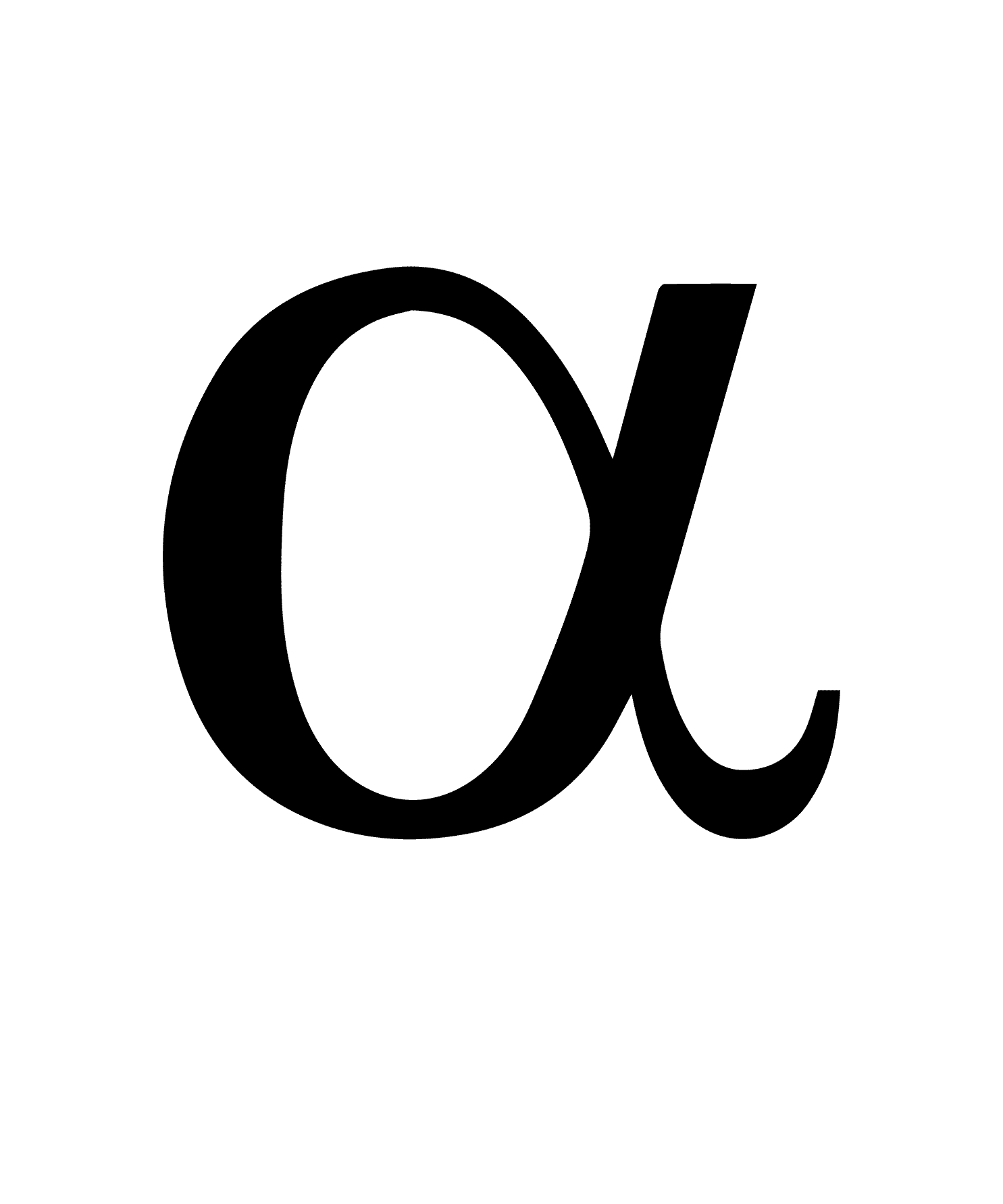
what does this symbol represent
Alpha particle or Alpha decay
A diagram representing just the Valence electrons of an element or compound
Lewis dot structure
When 1 or more elements or molecules switch places
AC + BD ----->AB + CD
Displacement reaction
Draw any alkali metal.
Include all parts of the atom with correct proportions and labels.
Answers vary
How many L are equal to 2500 cm3.
2.5 Liters
He was known for his gold foil experiment- fired negative ions at thin sheet of gold foil, discovered the atomic nucleus and proposed a nuclear model of the atom , who is he ?
Ernest Rutherford
Define half-life.
Name 1 use for half-lives.
The time required for one-half of a radioactive sample to decay.
Carbon dating, Medical - blood tracers, Handling nuclear waste
What type of bond is produced by unequal sharing electrons?
Polar-covalent bonds
Difference between synthesis and decomposition reactions.
Synthesis is putting 2 or more substances combine to form a new substance.
Decomposition is a compound breaking down into 2 or more simpler substances.
Opposite reactions.
How is avg atomic mass calculated?
Summing the masses of an element’s isotopes multiplied by each percent abundance.
Ex: Average atomic mass of chlorine
(0.75 x 35 amu) + (0.25 x 37 amu) = 35.5 amu
Give an example of an element, a compound, a heterogeneous and homogeneous mixtures?
Answers vary
JJ Thompson's model for the atom called?
You can have a hint for half-credit.
The Plum Pudding Model
33. Radium-226 decays by alpha decay to
A) barium-131. B) cobalt-60.
C) radon-222. D) polonium-218.
C
What is electronegativity? What type elements will have low electronegativity? What type of elements will have a high electronegativity?
The desire to gain electrons. Metals have low electronegativity and non metals have High electronegativity.
When fuel is burned what is created?
CO2 + H2O + ENERGY
All group 18 elements have a full valence electrons. This means the noble gases (__________________.
Won't react with other atoms.
How many atoms are in 45.0 moles of Zn(s).
Correct significant figures and unit label.
2.07 x 1025 atoms.
Who Was Democritus?
Greek philosopher that said all matter is made of tiny, indestructible particles called atoms
46. Sodium-24 has a half-life of 15 hours. How many hours is three half-lives?
A) 60 hours B) 45 hours
C) 30 hours D) 15 hours
E) 7.5 hour
B
What are 2 ways to identify a bond as ionic or covalent?
1. Determine types of elements that are bonding
m + nm = ionic
nm +nm = covalent
Electronegativity Difference greater than 1.7 = ionic
less than 1.7 = covalent
Include as many pieces of information as you.
C12H22O11 + H2O + yeast→ 4C2H5OH + 4CO2