A small particle that is the building block of matter
What is an atom?
A substance made of only one kind of atom
What is an element?
The subatomic molecules found in the nucleus of the atom.
What are protons and neutrons?
Baking brownies, baking pizza crust, and dying your hair are examples of this.
What are chemical changes?
NAC2H3O2, H2O, and CO2 are examples of this.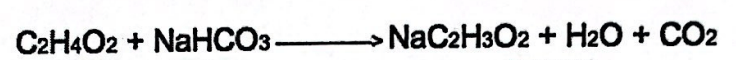
What are reactants?
What is a substance?
A mixture where the substances are NOT evenly mixed
These two things make up the atomic mass of an atom.
What are protons and neutrons?
Ice melting, cutting pepperoni, and ripping paper are examples of this.
What are physical changes?
C2H4O2, NAHCO3 are examples of this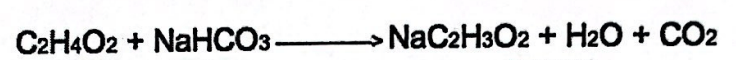
What are reactants?
Two or more atoms that are held together by chemical bonds and act as a unit
What is a molecule?
SO3 contains _______ sulfur and _______ oxygen.
What is 1 sulfur and 3 oxygen?
Draw the atomic model of Sodium.


A chemical change is either associated with compounds or mixtures.
What is a compound?
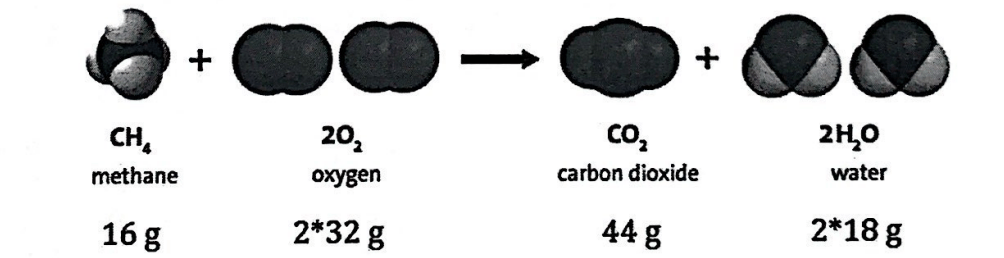
Find the mass of the reactants.
80 g
Many singular molecules that bonded together
What is an element?
A mixture where substances are EVENLY mixed, can also be called a solution
Homogenous mixture
Draw the atomic model of Oxygen.

Three signs of a chemical reaction.
Find the mass of the products. 
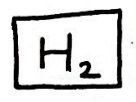
What is a diatomic molecule?
The smallest part of the element that still maintains the characteristics of that element.
What is an atom?
Why are electrons not included in atomic mass?
Electrons move too quickly
Electrons are too small
This happens to atoms during chemical changes.
What are atoms rearranging? / What are the rearranging of atoms?
In this system, the mass does not change.
What is a closed system?