What is the electron configuration for Li?
What is 1s^2 2s^1
Identify the independent and dependent variables:
Amount of calories burned, c, and the number of minutes, m, exercising
Calories = dependent
Minutes = independent
5004
4
To fill the 3d orbital, what orbital must be filled in first?
What is the 4s orbital
Which subatomic particles does the nucleus of an atom contain?
What element is [Ne] 3s^2 3p^5 ?
What is Cl
How many electrons can a s subshell have?
What is 2
Identify the independent and dependent variables:
tickets, t, sold for a race and the amount of money, m, collected
Tickets = independent variable
Money = dependent variable
140
2
How many neutrons are in a potassium (K) atom?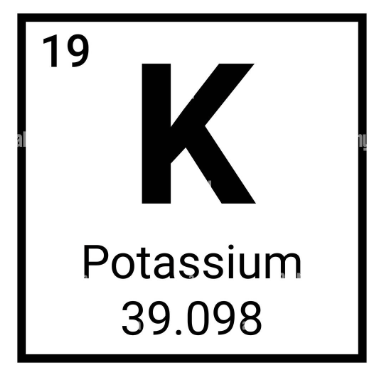
20
How many electrons can a p subshell have?
What is 6
The time it takes to run a mile depends on the person’s running speed.
Dependent = time it takes to run a mile
Independent = person's running speed
7,500,001,000
7
An atom that lost an electron is called a(n)
cation
What is the noble gas electron configuration of Ag?
What is [Kr] 5s^2 4d^9
In the Bohr model of nitrogen (N, atomic # = 7), the second energy level contains how many electrons?
What is 5
The higher the temperature of the air in the oven, the faster a cake will bake.
Dependent = speed cake bakes
Independent = oven temperature
12.0000
6
Write an equation to determine the average atomic mass of Li with the following isotope data: 6.017 u, 7.30%, and 7.018 u, 92.70%.
average atomic mass = (6.017 amu x 0.0730) + (7.018 amu x 0.9270)
How many electrons can the 4th energy level have? Think about electron configuration rules.
What is 2n^2 = 32
Students measured the temperature of the water at different depths in Lake Norman and found that the temperature varied.
Dependent = temperature of water
Independent = depth measured
0.0050010
5
Adding or subtracting neutrons makes the element change into an ...
isotope