Name this compound NH4Cl
Ammonium Chloride
How many protons does Lawrencium have
103
What is the symbol for Gadolinium
Gd
Alpha particles has this kind of charge
Positive
Your friend decides to toast a piece of bread, but leaves it in the toaster too long. The bread is black and the kitchen if full of smoke. Is this a chemical or physical change?
Chemical
What compound is lithium iodide
LiI
How many neutrons are in Europium?
89
What is the name of metals where you can find molybdenum?
Transition metals
The type of process nuclear power plants use
Fission
The teacher was not in the room yet. Jake began weighing chemicals, touching them with his hands. His nose itched so he rubbed it. What safety issues has Jake broken?
1. Touching lab without a teacher present
2. Touching chemicals with his hands
3. Not washing his hands
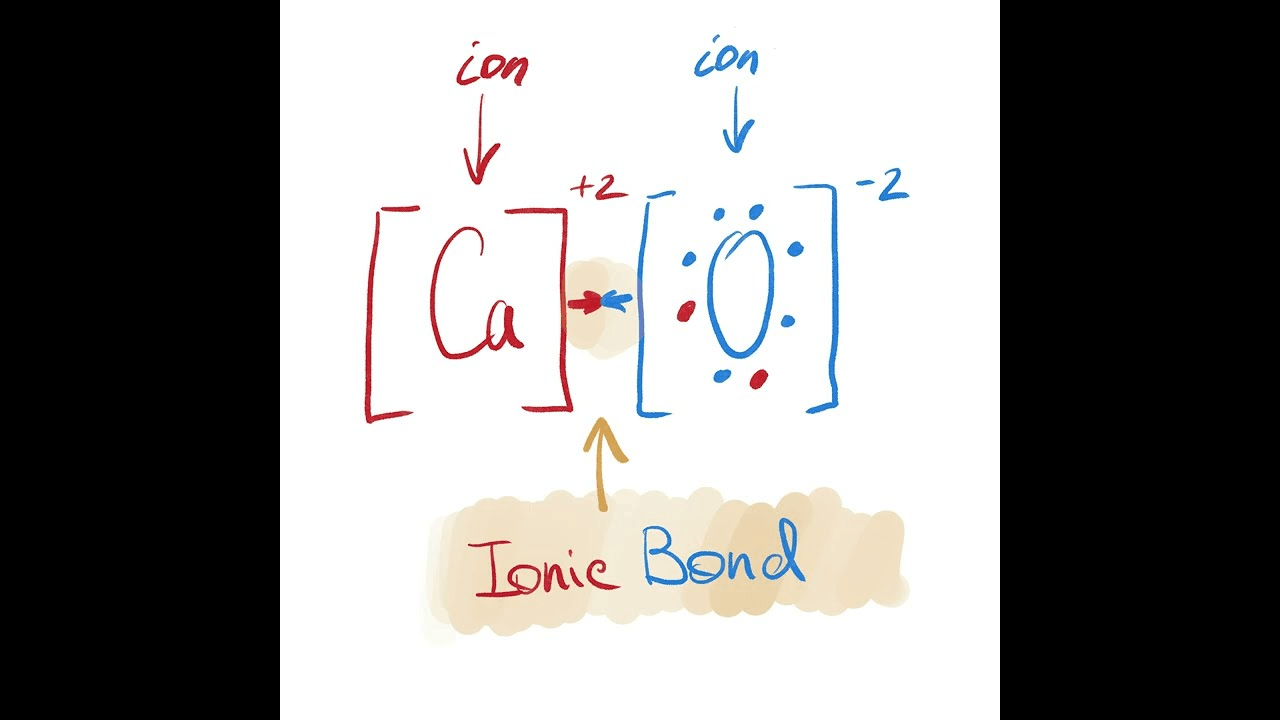
Draw the Lewis dot diagram of CaO
What is the electron configuration of Fe
1s22s22p63s23p64s23d5
Put these in order from highest atomic radii to lowest atomic radii: Ni, Fr, P, Au
Fr, Au, Ni, P
2211Na + _____________ -> 2210Ne
0-1e
How many electrons does Ag+2 have?
45 electronds
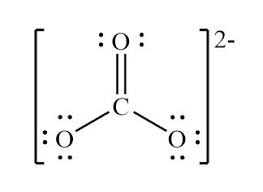
Draw the Lewis Dot Diagram of CO3-2
Ernest Rutherford, J.J. Thomson, or Schrodinger all contributed to our understanding of the atom. Choose one of the scientists listed above and explain his contribution to our current understanding of atomic theory and/ or atomic models.
Rutherford: Gold foil experiment, proved there was a nulceus that is positively charged
Thomson: Cathod ray experiment, proved atoms have electrons
Schrodinger: Fancy math, electrons move like bees around a beehive
What group, period and name of this element [Xe]6s24f145d106p5
Group: 17
Period: 6
Name: Astatine
Which particle is stopped by concrete or lots of water?
Gamma
Substances initially involved in a chemical reaction are known as what?
Reactants
Testing of an unknown solid shows that it has the properties listed below. (1) l.ow melting point (2) soluble in water (3) poor conductor of electricity (4) relatively soft solid
State the type of bonding that would be expected in the particles of this substance,
Covalent
Given the relative abundance of the following naturally occurring isotopes of oxygen, calculate the average atomic mass of oxygen. Assume that the atomic mass of each is the same as the mass number. oxygen- 16: 99.76% oxygen- 17: 0.037% oxygen-18: 0.204%
16.00 AMU
How is electronegativity enery different from ionization energy?
electronegativity -> How easy it is to accept an electron
Ionization -> Energy required to get rid of an electron
The half-life of cobalt-60 is 10.47 min. How many grams of cobalt-60 remain after 104.7 min if you start with 1024g?
1g
Balance the following equation:
Fe2O3(s) + C(s) ---> Fe(s) + CO2(g)
2Fe2O3(s) + 3C(s) ---> 4Fe(s) + 3CO2(g)