This is the name of an atom that has gained or lost electrons
ion!
This type of reaction is formulated like this:
AB-> A + B
Decomposition
This is the name used to describe the concentration of something aka "moles per liter"
molarity or M
This is the weakest intermolecular force, also known as "London" or "VanDerWaals"
Dispersion force
A substance with a pH of 8 would be classified as a ...
base
This is the name of a bond between two non-metals
covalent!
This type of reaction involves two atoms switching places
double replacement
True/false: A chemist has in his laboratory a 12M hydrochloric acid solution. In this solution there are 12 grams of HCl per liter of water
False
polar
this is defined as a substance that loses a Hydrogen proton
acid
This type of bond has the greatest difference in electronegativity
ionic bond!
This type of reaction always results in the formation of a water molecule
combustion
This number, also known as avogadro's number, represents one mole:
6.02 x 10^23
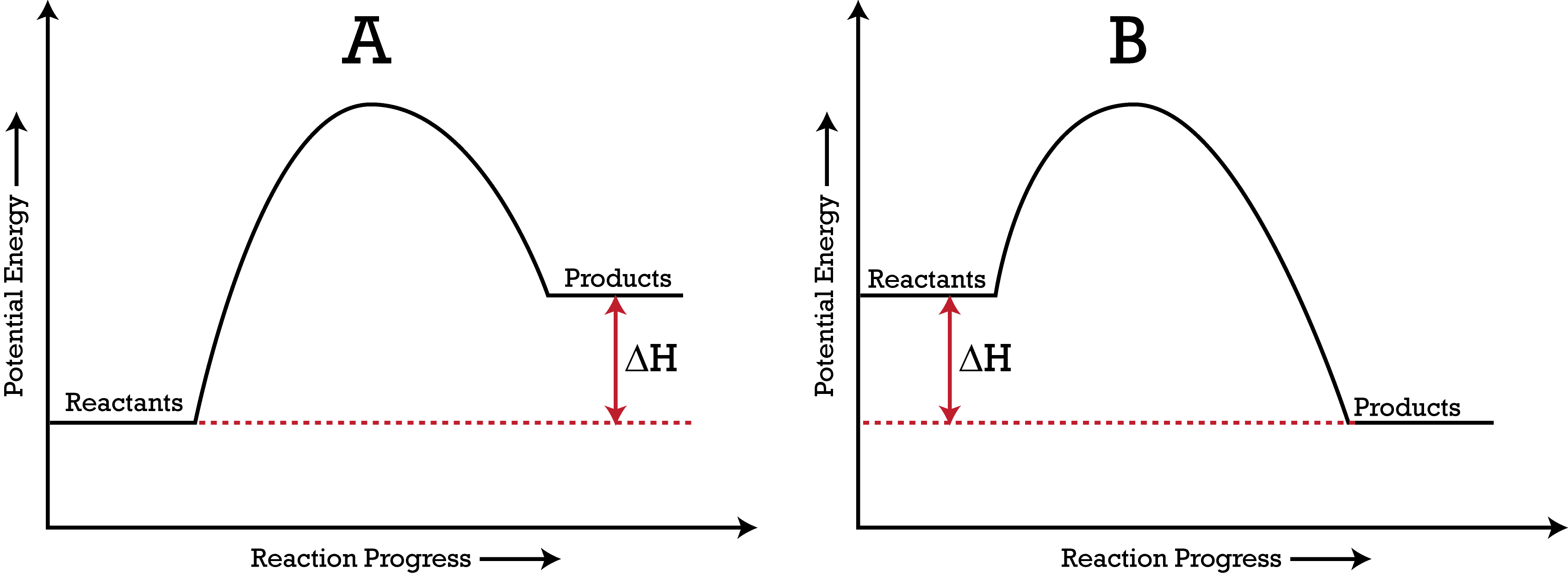
Which is the endothermic reaction?
A
a substance with a pH of 2 is very likely to do what in water
The elements in the halogen family (beginning with fluorine) will _(gain/lose)____ this many electrons to make an octet
a balanced version of this equation would look like...
Fe2O3 + Al → Fe + Al2O3
Two moles of carbon would weigh this much: 
about 24 grams
water!
H3O (hydronium) is a common product of water as a base accepting a hydrogen. This is called a ____ acid
conjugate
Oxygen will be drawn with this many valence electrons:

6
DAILY DOUBLE:How many electrons are being shared in an entire molecule of water? Draw it out to check!
4
How many grams of Hydrogen are in 20 moles of hydrogen? (round down)
20 grams
This type of "bond" is actually not correct, it's just a very strong form of dipole-dipole force
hydrogen bond
HNO3 + H2O would result in these two products
H3O(aq)+NO3-