Subatomic particle that orbits the nucleus
What is the electron?
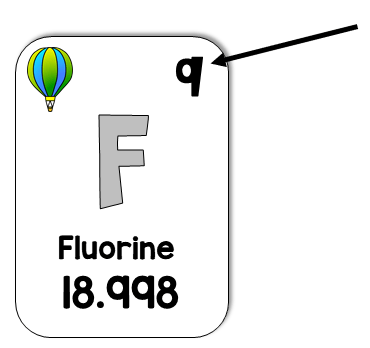
What is the atomic number?
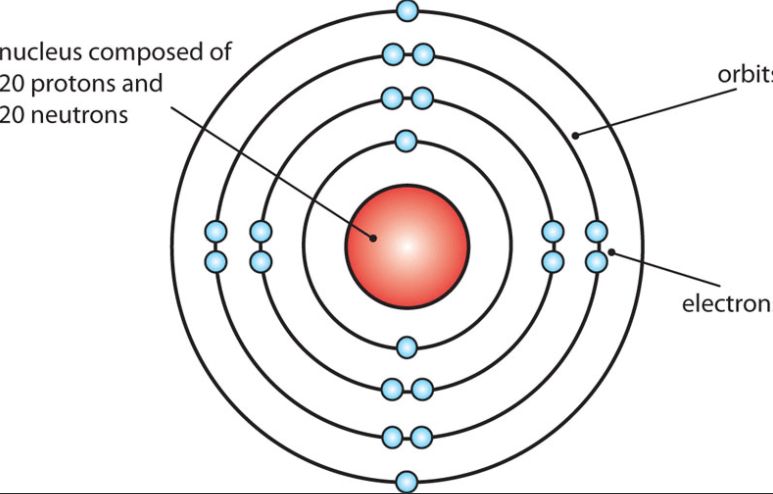
What is calcium?
He is the inventor of the periodic table.
Who is Mendeleev?
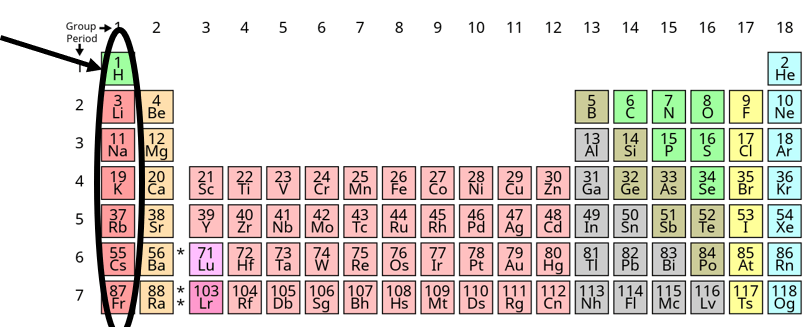
What are the Alkali metals?
They're the elements that form ionic bonds.
What are metals and non-metals?
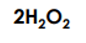 Has this many molecules.
Has this many molecules.
What is 2?
It's the law that says that during a chemical reaction, matter is neither created or destroyed.
What is the Law of Conservation of Matter?
It's what the Nucleus is made up of.
What are protons and neutrons?
It's what the atomic number counts.
What is the number of protons?
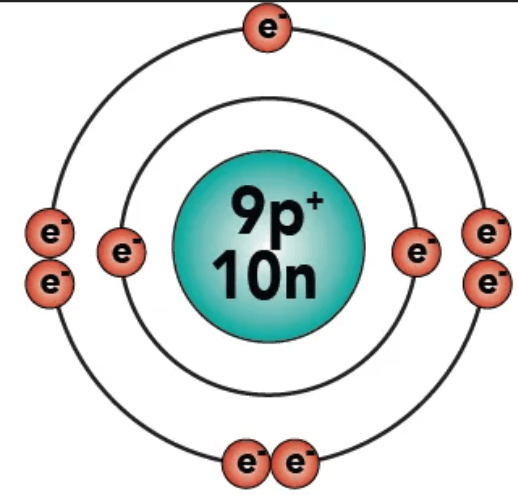
What is Fluorine?
It's how many groups/families are on the periodic table.
What is 18?
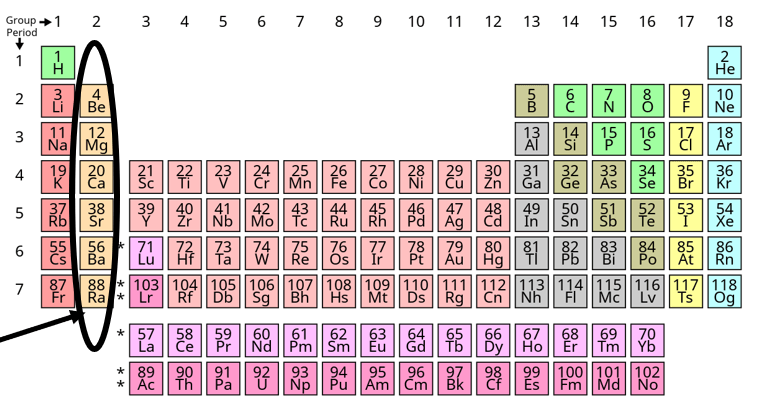
What are the Alkaline Earth Metals?
They're the elements that bond with covalent bonds.
What are non-metals with non-metals?
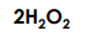
Has this many atoms.
What is 8?
It's what this part of the chemical reaction is called.
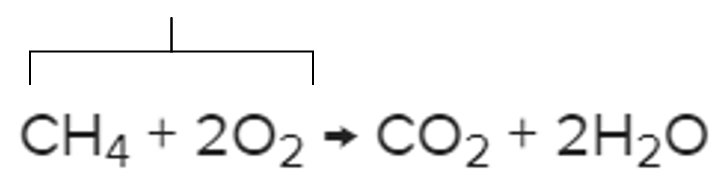
What are the reactants?
Its the charge that electrons have.
What is a negative charge?
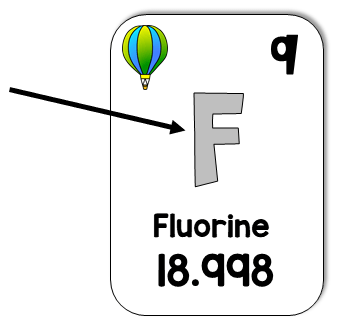
What is the element symbol?
It is the number of electrons that oxygen has.
What is 8?
It's the number of periods/rows are on the periodic table.
What is 7?
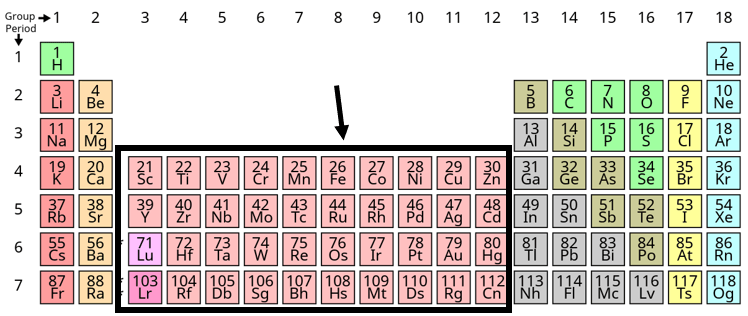
What are the Transition Metals?
It's how ionic bonds are formed.
What is, electrons are given or taken?

Has this many molecules.
What is 3?
It's what this part of the chemical reaction is called.
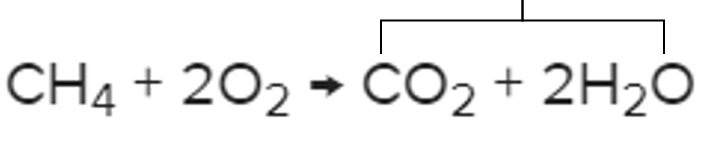
What are the products?
It's the charge that protons have.
What is a positive charge?
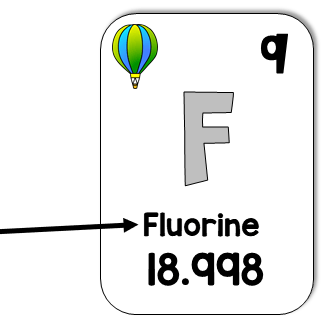
What is the element name?
It's the number of neutrons chromium has.

What is 28?
Electrons that are found on an incomplete outer shell.
What are valence electrons?
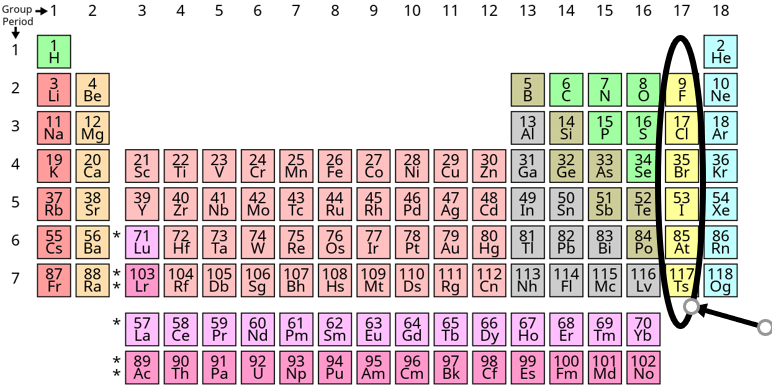
What are the Halogens?
It's how covalent bonds are created.
What is, electrons are shared between atoms?

Has this many elements in it.
What is 2?
Balanced or unbalanced?
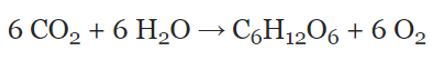
What is balanced?
It's the charge that neutrons have.
What is a neutral charge?
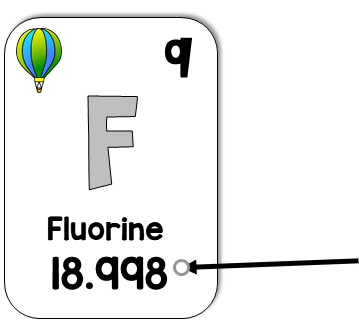
What is the atomic mass?
It is an element whose protons and neutrons are not the same number.
What is an Isotope?
It's how you can tell how many electron shells an element has.
What is by the period number?
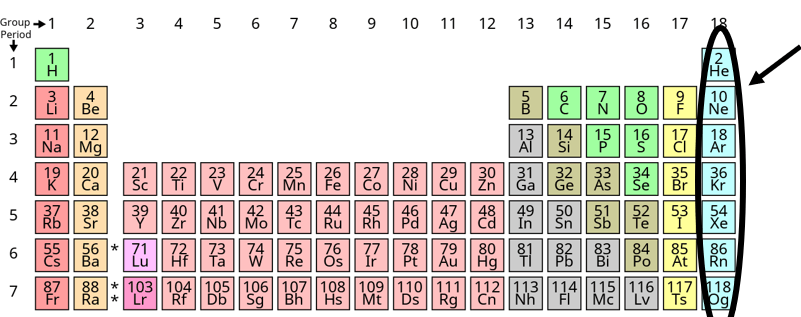
What are the Noble Gasses?
It's the type of bond in an HCl molecule.
What is a covalent bond?

Has this many total atoms.
What is 15?
Balanced or unbalanced?
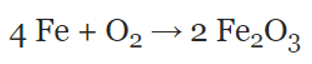
What is unbalanced?