1 mole = ______ L
22.4 L
Write the formula for: Oxygen pentafluoride
OF5
Name the 5 types of reactions
single replacement, double replacement, combustion, decomposition, combination
When 2.85 moles of oxygen reacts with excess magnesium, how many moles of magnesium oxide are formed?
2Mg + O2 --> 2MgO
5.70 moles MgO
Standard temperature is equal to ____K
273 K
the pH of lemon juice is 2.5.
1) is it basic, neutral, or acidic?
2) what is it's pOH?
1) acidic
2) 11.5
What is the mass of 0.20 moles of Ga2(SO3)3?
76g
Name the ionic compound: CuF2
copper (II) flouride
Convert the word equation into a formula equation (don't balance):
barium chlorate → barium chloride + oxygen
Ba(ClO3)2 → BaCl2 + O2
If 146.5 g of Chromium(III) chlorate decomposes, what mass of oxygen can be produced?
2 Cr(ClO3)3 --> 2 CrCl3 + 9 O2

A rigid container holds a gas at a pressure of 55kPa and a temperature of -100.0°C. What will the pressure be when the temperature is increased to 200.0°C?
P = 150 kPa
(55/173)=P/437
What is the molarity of a solution that contains 4.5 moles of NaCl in 3800 mL of solution?
4.5/3.8 = 1.18M
What mass of carbon monoxide gas (CO) would occupy a volume of 4.30 L at STP?
5.38 g CO
The molecular compound Se8O7 is named...
octaselenium heptaoxide
Predict the product (in words)
Barium + Aluminium nitrate -->
Barium + Aluminium nitrate --> Barium nitrate + aluminium
In an experiment, 170.9 g of C2H4 was reacted with an excess of O2, 164.1 g of CO2 is produced.
C4H4 (g) + 3 O2 → 2 CO2 + 2 H2O
What is the percent yield of this reaction?
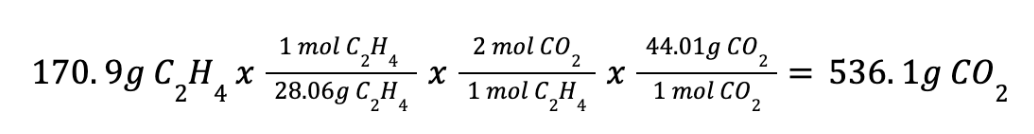
(161.1g / 561.1)*100 = 30.61%
The following have a _____ relationship (direct or indirect)
1) pressure and temperature
2) volume and pressure
3) pressure and volume
1) direct
2) direct
3) indirect
Calculate the pH of a solution with H+ of 1*10-7
Is the solution basic, neutral or acidic?
pH =7
neutral
What is the % composition of carbon in CH4?
77.9%
What is the name of this acid? H2(CO3)
carbonic acid
balance the equation:
_____ Al + _____ H2O → _____ Al(OH)3 + _____ H2
2 Al + 6 H2O → 2 Al(OH)3 + 3 H2
If 13.5 L of nitrogen gas reacts with 17.8 L of hydrogen gas at STP, according to the following reaction, what mass of ammonia would be produced?
What is the limiting reactant
N2 + 3H2 --> 2NH3
Hydrogen 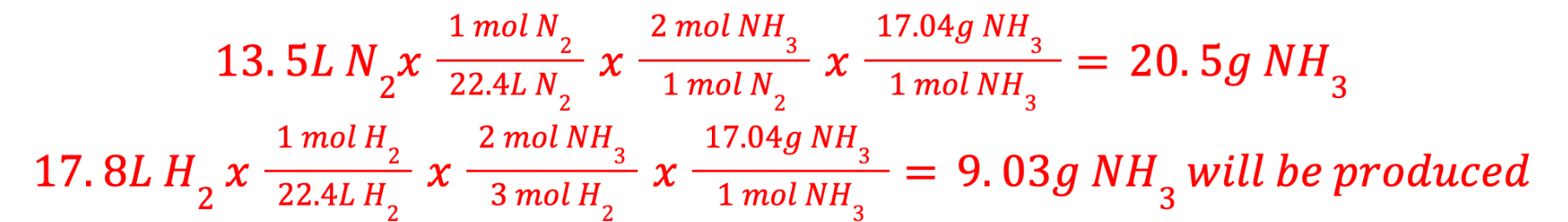
What is the pressure of 0.33 moles of nitrogen gas, if its volume is 15.0 L at –25.0 C?
include the unit!
P = 0.45 atm
Calculate the [OH-] of a substance with a pOH of 8.5
3.16*10-9 M