Based on the picture below, write out two formulas for Elemental Molecules: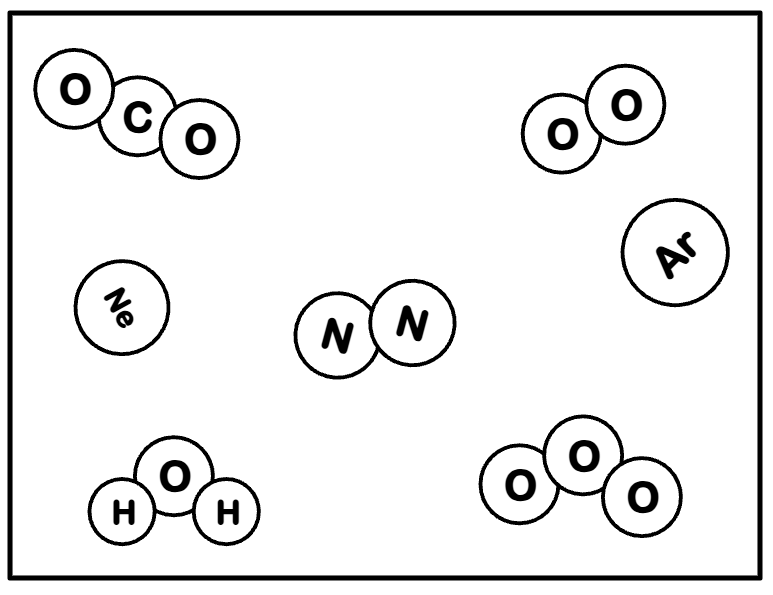
O2 , O3 , and N2
What are the 3 main subatomic particles in an atom?
Protons, Neutrons, and Electrons.
Which type of compound needs to use prefixes in the name: Ex: Carbon Dioxide and Carbon Monoxide for CO2 and CO
Molecular (aka Covalent) Compounds
What are the two sources to get the numbers for your conversions from?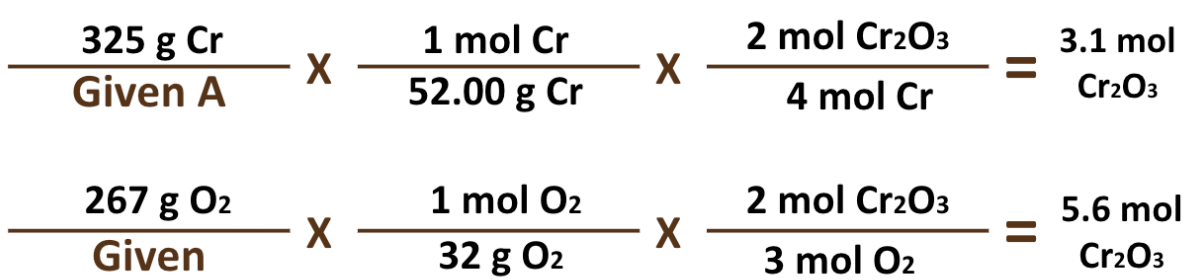
(1) The Periodic Table (for molar masses g/mol) and (2) Balanced Equations Ratios(for mole to mole relationships to switch from one chemical to another chemical)
1 N2 + 3 H2 --> 2 NH3
Identify the subscripts and the coefficients in the above reaction
1:3:2 are the Coefficients, the 2 2 3 are the subscripts.
Write out the two formulas for the Compounds found in the picture below:
CO2 and H2O
What is the center of the atom called and which particles are inside of it?
The nucleus contains both protons and neutrons (both of which are made of quarks)
1:4 ratio = TiCl4
What is the Theoretical Yield for the attached problem? (Include #, unit, and chemical)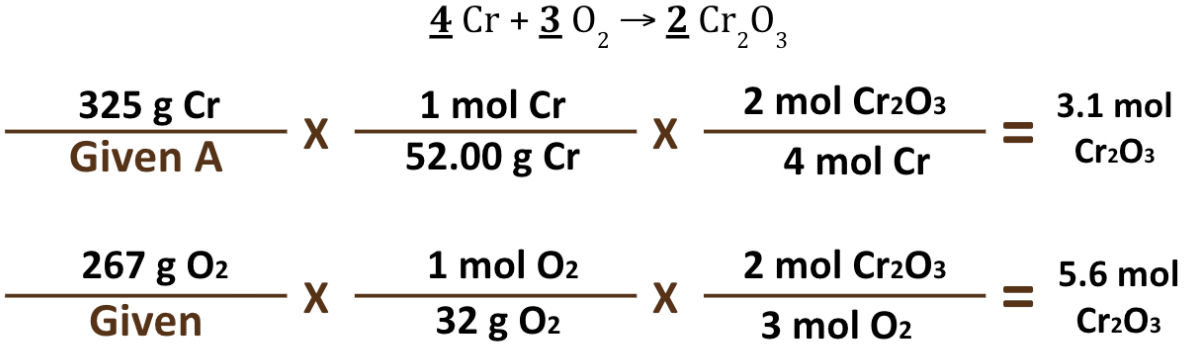
3.01 mol Cr2O3
1 N2 + 3 H2 --> 2 NH3
In the above reaction, how many Hydrogens are on the product side?
2 NH3 = 6 Hydrogens
What is the difference between a Pure Group and a Mixture? What would the following group of substances be considered:
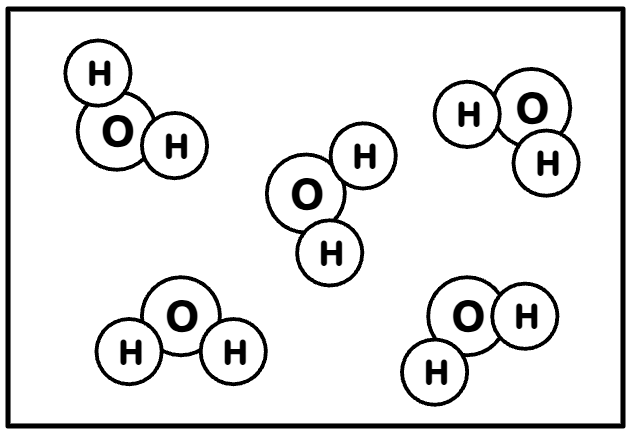
Pure = all the same, Mixture = different types of substances.
This is pure because they are all H2O
What element/ion has 17 protons, 18 electrons, and 19 neutrons?
Chlorine (This Chlorine has a -1 charge and is also known as Chloride or the Chloride Ion)
What is the name of the following compound: (NH4)3N
Ammonium Nitride
Identify the Limiting and Excess Reactants in the following problem: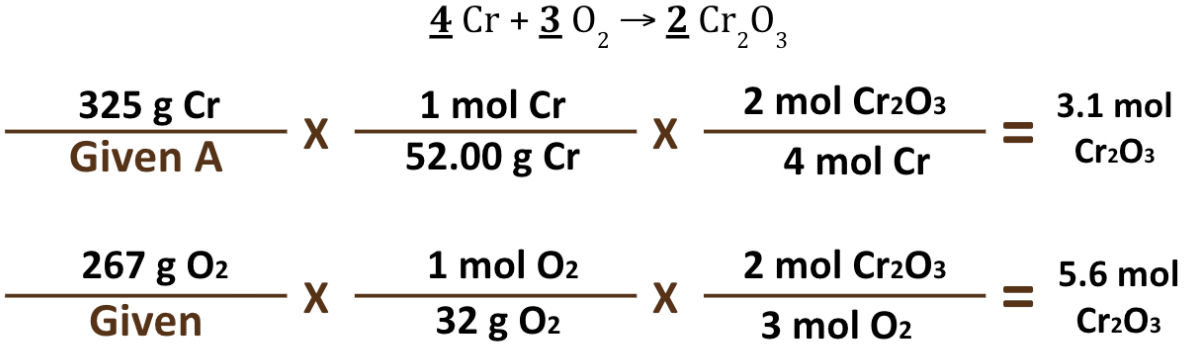
Cr = Limiting (it can only make 3.1 moles of the product)
O2 = Excess (It can make up to 5.6 moles of the product, but it is limited to only making 3.1 moles and then those other Oxygen molecules will be leftover/not used)
C3H8 + ___ O2 --> 3 CO2 + 4 H2O
How many oxygens are on the product side of the above reaction? What coefficient should go in front of the O2?
10 Oxygens (5 from CO2, and 4 from H2O)
5 O2 = 10 Oxygen atoms
If a substance is found to have an empirical formula of CH2 and has the following Mass Spectrum (showing the molar mass of the actual substance), what is the actual Molecular Formula of the compound?
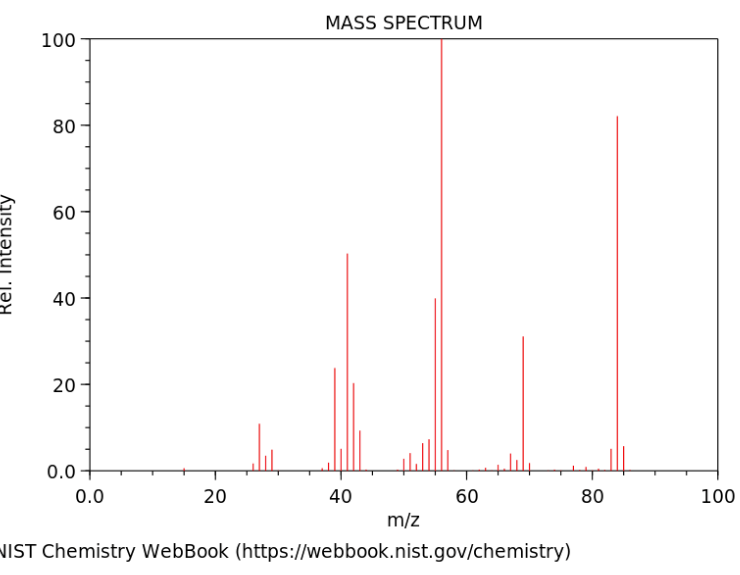
C6H12
Molar Mass from the graph is about 85 grams. CH2 = 14g, 14x6 = 85 grams
If an atom has 38 protons, 36 electrons, and 40 neutrons, what is its Mass Number and Charge?
Mass number = 38+40 = 78 amu
Charge = +38 -36 = 2+
What is the formula for the Ionic Compound: Iron(III) Sulfate
Fe2(SO4)3
Based on the mole answers, figure out how many grams of product should theoretically be produced:
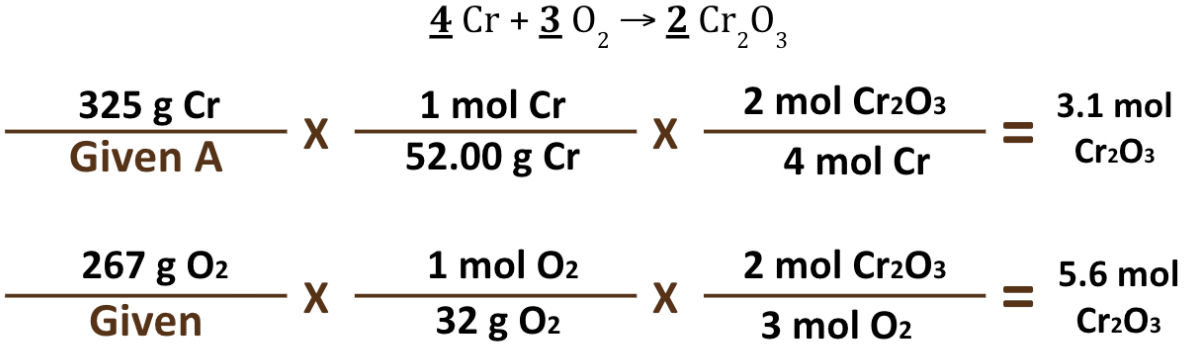
471.26 grams of Cr2O3
(Molar Mass of Cr2O3 = 152.02 g/mol)
(3.1 mol x 152 g/mol = 471.26 g Cr2O3)
__B2Br6 + __HNO3 --> __B(NO3)3 + __HBr
Balance the above reaction.
1 B2Br6 + 6 HNO3 --> 2 B(NO3)3 + 6 HBr
1:6:2:6
How many valence electrons does the following element have? What will it do to become stable?
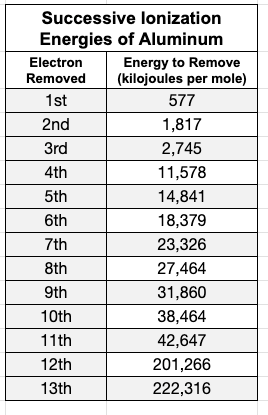
3 valence electrons, and will lose all 3 to become stable.
Identify how many protons and neutrons are in the two stable Isotopes shown in a pure element's mass spectrum below: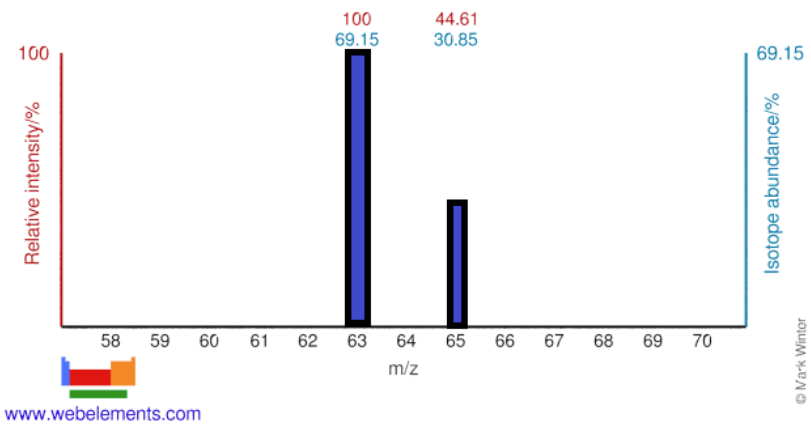
The element is Copper, so they both have 29 protons, but the light Isotope has 34 neutrons (29+34 = 63) and the heavier Isotope has 36 neutrons (29+36 = 65)
What is the name for the following compound: Mn(Cr2O7)2
Manganese(IV) dichromate
When gathering Copper from the Copper Nail, a group should have theoretically gather 6.1 grams of Copper. If in reality they only gathered 5.5 grams of the final product, what is their Percent Yield, and what could have resulted in the 0.6 g deficit?
90% = 5.5/6.1*100
Loss during separation (maybe filter paper or decanted too fast)
Equilibrium Restrictions
Side Reactions
Impurities causing inaccurate predictions
__K(OH) + __H3PO4 --> __K3PO4 + __H2O
Balance the above reaction.
3 K(OH) + 1 H3PO4 --> 1 K3PO4 + 3 H(OH)