Fill in the blanks with ionic or covalent:
1. ______ bonds are formed between a metal and a nonmetal.
2. ______ bonds are formed between 2 nonmetals
1. ionic
2. covalent
Why do some liquids "stick" together more than other liquids? What is an example of a liquid with high surface tension?
Water is very "sticky," giving it high surface tension.
Identify an example of a carbon SOURCE and a carbon SINK.
Answers will vary- up to teacher discretion
Possible sources: volcanic eruptions, burning of fossil fuels, respiration
Possible sinks: photosynthesis, fossil fuel formation
THIS type of reaction takes IN heat, therefore making the surroundings COLDER.
endothermic reaction
Can a wooden spear that is 25,000 years old be dated using carbon-14? Explain.
Yes! This sample was once alive (is organic) and is less than 35,000 years old.
What is the difference between an ionic and a covalent bond? (Hint: it has to do with what happens with the electrons between atoms!)
In an ionic bond, the electrons are transferred from one atom to another, creating one positively charged ion (cation) and one negatively charged ion (anion). In a covalent bond, the electrons are shared between atoms.
What is evaporative cooling?
Evaporative cooling is the phenomenon that explains how a surface gets colder as a liquid evaporates.
Explain what the law of conservation of mass states, and how we can use this to balance chemical equations.
The law of conservation of mass states that mass/ matter cannot be created nor destroyed. Therefore, when we balance chemical equations, we MUST have an equal amount of all atoms on the left and right sides of the equation.
THIS type of reaction RELEASES heat, therefore making the surroundings HOTTER
Exothermic reaction
What is the symbol for an alpha particle?
4/2 He
Can ionic compounds (for example, salt, NaCl) conduct electricity? Explain.
Yes, ionic compounds dissolve into ions in solution and are able to conduct electricity. Covalent compounds remain intact and do not dissociate into ions, and therefore do not conduct electricity.
In terms of intermolecular forces, why does acetone evaporate so much faster than water?
Acetone has much WEAKER intermolecular forces than water, allowing the molecules to break away from each other and evaporate faster.
Balance the following chemical equation:
NaF + Br2 --> NaBr + F2
2NaF + Br2 --> 2NaBr + F2
Water has a very high specific heat, meaning that it takes a lot of energy to heat it up. Sand has a lower specific heat, so it does not take very much energy to heat up.
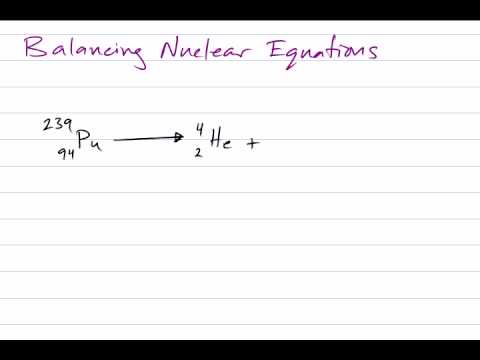
Balance this nuclear equation.
235/ 92 U
Name EACH of the following compounds:
1. MgO
2. CO
1. Magnesium oxide
2. Carbon monoxide (prefixes are needed because this compound is covalent)
How do the temperature and the kinetic energy of a liquid change during the evaporation process?
The particles that are evaporating have higher temperatures and kinetic energy, leaving the particles with lower temps and lower kinetic energy behind. Therefore, the liquid that remains gets colder.
Name the reaction type:
KBr + AgNO3 --> KNO3 + AgBr
Double replacement reaction
Use the equation Q=mCT to solve the following problem:
If the temperature of 34.4 g of ethanol increases from 25 °C to 78.8 °C, how much heat has been absorbed by the ethanol? The specific heat of ethanol is 2.44 J/(g×°C).
4,515.8 Joules
Where does nuclear fission occur? Where does nuclear fusion occur?
Write the molecular formula for the following ionic compound: calcium chloride and the following covalent compound: diphosphorous tetrafluoride.
CaCl2, P2F4
Explain why water is considered to be a polar molecule.
Write a word equation for the following chemical reaction (including states of matter!)
CaCO3 (s) --> CaO (s) + CO2 (g)
Solid calcium carbonate reacts to form solid calcium oxide and carbon dioxide gas.
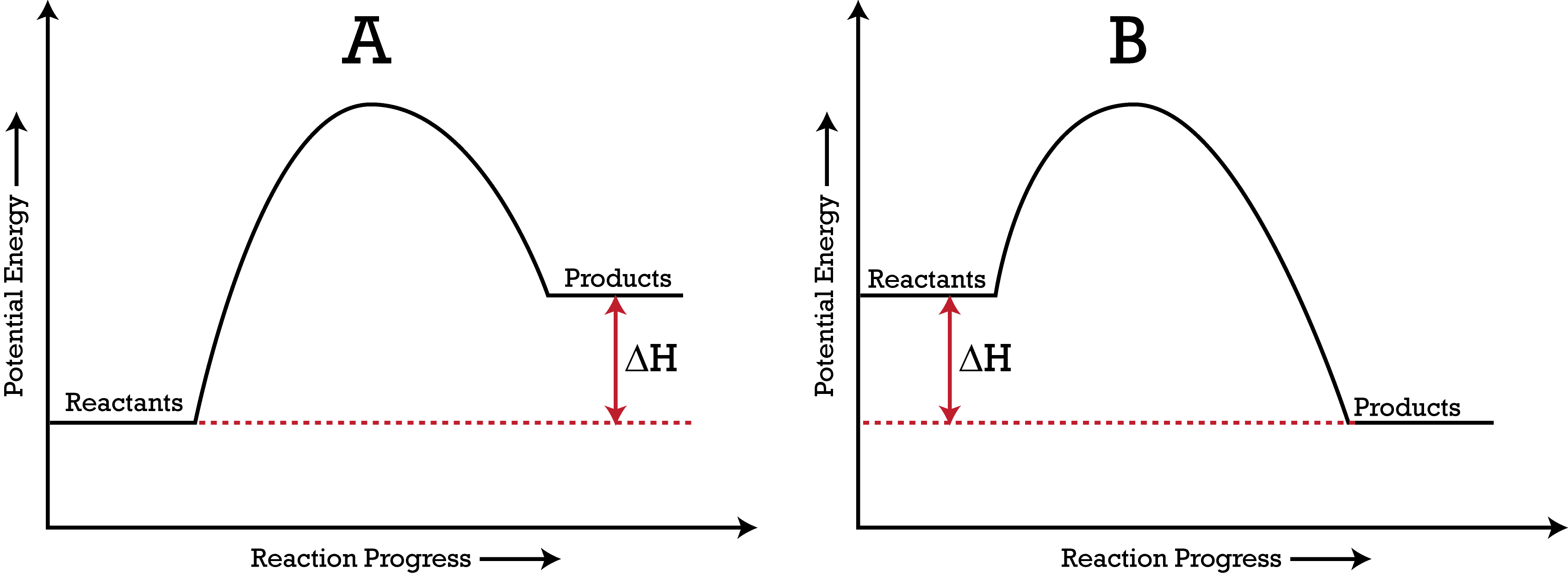
Based on these energy diagrams, which reaction is endothermic, and which reaction is exothermic?
Left: Endothermic because the energy of the products is greater than the energy of the reactants.
Right: Exothermic because the reaction loses energy and the energy of the products is less than the starting energy of the reactants.
Nitrogen-13 has a half life of 10 minutes. How many minutes will it take for a radioactive sample to decay from 4,000 grams to 250 grams?
40 minutes (4 half lives have passed)