This is the number of valence electrons for the metals in group 13.
What is 3?
These particles make up the majority of the mass of an atom.
What are protons and neutrons?
This is the number of valence electrons in the compound H₂O.

What is 8?
This is the molar mass of AgCl₃, with units.
What is 214.22 g/mol
What is 400 K?
In this chart, this is the type of radiation that has the highest frequency.
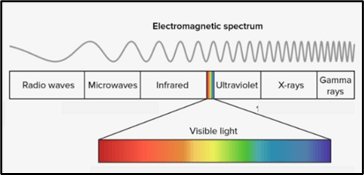
What are gamma rays?
80% of the elements on the periodic table are this type, which are typically shiny, malleable, and good conductors of electricity.
What are metals?
The compound PF₅ is made up of only nonmetals, which means it's this type of compound, where electrons are shared.
What is covalent?
What is 3?
Out of saturated, unsaturated, and supersaturated, a solution that contains visible solute at the bottom can be described as this type.
What is saturated?
This is the hyphen notation for the isotope shown below.
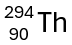
What is thorium-294?
This group on the periodic table has the greatest ionization energy.
What are the noble gases?
or
What is group 18?
Since the compound CuCl₂ is made up of a metal and a nonmetal, the electrons will be transferred from copper to chlorine and it would be classified as this type of compound.
What is ionic?
This is the mass of 3.0 moles of silicon dioxide (SiO₂), including units.
What is 180 grams?
When a 2.00 M solution of HCl is diluted from 0.200 L to 3.00 L, this is its new concentration.
What is 0.133 M?
This is the mass number for an isotope of sodium with 13 neutrons.
What is 24?
This is the electron configuration for argon.
What is 1s² 2s² 2p⁶ 3s² 3p⁶?
This is the correct Lewis structure for methane, CH₄.
What is  ?
?
In this reaction pictured, these molecules are the limiting reactant.
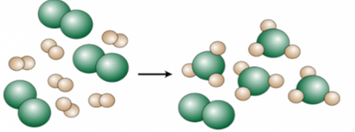
What are the white diatomic molecules?
This is the concentration of a 1.8 L sample of a solution with 28 grams of dissolved sodium hydroxide (NaOH), units included.
What is 0.39 M?
This is the result when americium-241 undergoes alpha decay by emitting an alpha particle.
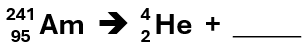
What is neptunium-237?
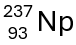
These types of particles determine the chemical properties of an atom. (Be specific!)
What are valence electrons?
This is the correct Lewis structure for carbon monoxide, CO.
What is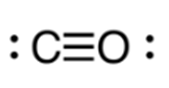 ?
?
If 6.0 moles of Cl₂ gas reacts with excess iron (Fe), this is the mass of FeCl₃ will be produced, including units.
2 Fe + 3 Cl₂ → 2 FeCl₃
What is 650 grams?
When a balloon 1.0 L in size is cooled from 30°C to 15°C, this will be the new size of the balloon, units included.
What is 0.95 L?