This type of compound uses prefixes in the name
As a solid this type of bonding is conductive
What is a metallic bond
When naming an ionic compound with a transition metal, this symbol is often used in the name
What is a Roman numeral
This is the name of the substances found on the left side of an arrow in a chemical equation
What is/are reactants
If I had a mole of dollar bills, I'd have this many dollar bills
What is 6.20 x 1023 dollar bills
What is my name? PCl3
What is phosphorous trichloride
This type of compound typically has lower melting and boiling points
what is a molecular compound
DOUBLE POINTS
When electrons are shared unequally between atoms, it is referred to as this type of bond?
What is a Polar covalent bond
This is the coefficient in front of chlorine in the following reaction
CH4 + Cl2 --> CCl4 + HCl
what is a 4
In order to convert from grams to mole or moles to grams, this value is needed
What is molar mass
DOUBLE POINTS
What is my formula: Calcium Oxide
What is CaO
When dissolved in water, this type of compound conducts electricity
What is an ionic compound
Which of these compounds would have stronger intermolecular forces. Liquid H2O or gaseous CO2
What is liquid H2O
When touching a beaker in which this reaction is occurring in, it will feel "cold" to the touch
what is an endothermic reaction
DOUBLE POINTS!
If you had 36 grams of water, about how many moles of water do you have?
What is 2 moles of water
What is the name of this compound: FeCl3
What is Iron (III) Chloride
This type of compound has different types of intermolecular forces that exist between molecules
What is a molecular compound
These 2 factors affect the strength of an ionic bond
What is distance and charge
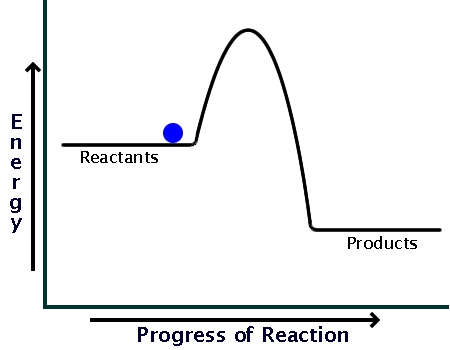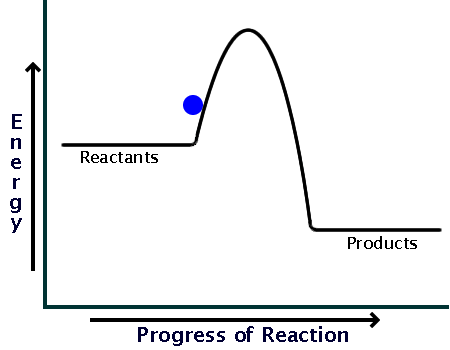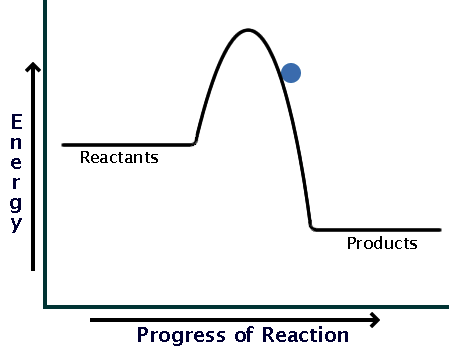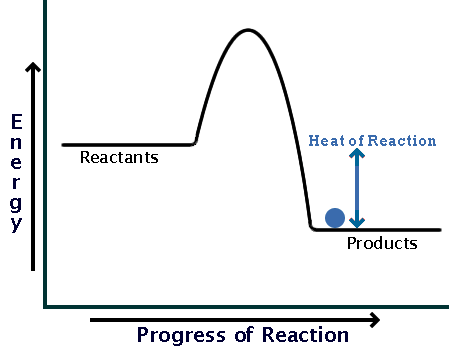
This energy profile represents which type of reaction
what is an Exothermic reaction
An unknown compound was analyzed and found to have a mass of 92 g/mol. If the empirical formula is NO2, what is the molecular formula?
What is N2O4
What is the formula for this compound: Nickel (II) Nitrate
What is Ni(NO3)2
This structure is responsible for the geometric shapes created by ionic compounds
What is a crystal lattice structure
Which of these compounds would have a higher melting point?
MgCl2 vs. CCl4
What is MgCl2 (ionic has higher melting points than molecular)
This would be an example of a "sign" that a chemical reaction has taken place
What is temp change, light, bubbles, unexpected color change
If the molar mass of CaCO3 is about 100 grams, what is the percent by mass of carbon in this compound?
what is 12% C