What is a cation?
A positively charged ion.
 What is D representing?
What is D representing?
Trough
London dispersion, dipole-dipole interaction, and ion-dipole interactions are all categories of which force?
Intramolecular or Intermolecular?
Burning your homework in a fire outback...
Is this an example of a chemical or physical property?
Chemical
What do the parts q = cm (delta) t mean?
q= joules
c = specific heat
m= mass
delta t = change in temp
What is the charge of lithium?
+1 Charge
He : ? :: H2S : Compound
Atom
Element Finish the analogy.
Cation
Anion
Answer: Element
Reasoning: Just plain He is an element on the periodic table but H2S is a compound, specifically known as Hydrogen Sulfide.
What is a physical property of a metallic bond?
Strength, Thermal and Electrical conductivity
Any closed system of mass will remain constant over time..
What is this defining?
The law of conservation of mass
Do you always show units and where they move to when solving a mole equation?
DUH
What was Rutherford's experiment?
Rutherford designed an experiment to use the alpha particles emitted by a radioactive element.
What is the difference between ground state and excited state?
Ground state: Lowest allowed energy state of an atom, molecule or ion.
Excited state: electron has moved to a higher energy level
When are roman numerals used in bonding?
_ Fe203 + _ C ---> _ Fe + _ Co2
2, 3, 4, 3
What heat transformation happens when you touch an ice cube?
Heat from hand will melt the ice
What is the electron configuration for Sodium?
1s^2 2s^2 2p^6 3s^1
What element is this representing? 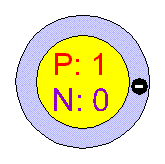
What is H202?
Dihydrogen Dioxide
2Co + 02 ---> 2Co2
What kind of reaction is this?
Synthesis
A + B = AB
What is the difference between calories and Calories?
Calories are the total amount of little calories
What is the difference between nuclear fission and fusion?
Fission: Taking one atom and splitting it into two atoms
Fusion: Two or more nuclei collide and join to form a new nucleus
What is this picture representing?

Electron dot configuration for Helium.
What are 2 of the diatomic molecules?
Hydrogen and nitrogen
Predict the products for...
Ca + CuCl2 ---> ??
CaCa2 + Cu
Heck yes