Covalent molecules are made up of (metal/nonmetal) atoms.
Covalent molecules are made up of nonmetal atoms.
What is an atom that has either gained or lost an electron?
An ion
What shape do 2-atom molecules take?
Linear
All reactions have __________ & products.
All reactions have reactants & products.
What is Avogadro's number (NA)?
NA = 6.02 x 1023
How many grams are in a 1 mole sample of carbon-12?
12 grams of carbon-12
A single covalent bond is formed when atoms share ___ valence electrons.
A single covalent bond is formed when atoms share two valence electrons.
Which type of ion completely donates its lone electron(s) during ionic bonding?
A cation
Which shape is seen in molecules with 4 noncentral atoms and 0 unshared electron pairs?
Tetrahedral
The Law of ____________ of Matter states that in a chemical reaction, matter cannot be created or destroyed.
The Law of Conservation of Matter states that in a chemical reaction, matter cannot be created or destroyed.
What do moles measure?
Number or Quantity of particles
In Chemistry, what does STP stand for?
Standard Temperature & Pressure
________ covalent bonds are formed when there is an EN difference less than 0.4
Nonpolar covalent bonds are formed when there is an EN difference less than 0.4
Which type of bond forms between two atoms who have an EN difference of 2.5?
An ionic bond
Polar compounds always have a ________ pole where electrons gather.
Polar compounds always have a negative pole where electrons gather.
What type of reaction is shown below?
N2 + 3H2 → 2NH3
Synthesis reaction.
________ mass for a substance is the amount of mass in 1 mole of that substance.
Molar mass for a substance is the amount of mass in 1 mole of that substance.
If the molar mass of Ca is 40 g/mol, then how many moles of calcium are found in a 80g sample?
(80g)/(40 g/mol) = 2 moles
What is the name of the molecule described below?
P2O5
Diphosphorus pentoxide
What is the overall charge of an ionic compound?
Zero (or neutral)
Is the molecule seen below polar or nonpolar?
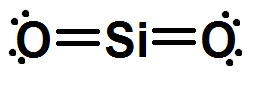
BONUS:
Explain why or why not.
Nonpolar.
Reason: There are 2 negative poles.
A _______-replacement reaction occurs when two ionic compounds trade ions.
A Double-replacement reaction occurs when two ionic compounds trade ions.
Molar _________ is the amount of space 1 mole of a gas (at Standard Temp & Pressure) occupies.
Molar Volume is the amount of space 1 mole of a gas (at Standard Temp & Pressure) occupies.
How many moles of a gas (at STP) are in a 22.4 L sample?
1 mole
What is the formula for the following molecule?
Sulfur hexafluoride
SF6
What is the charge of the iron cation found in the compound below?
FeO
Bonus: Explain your answer
2+
Reason: Oxygen forms an ion with charge 2- & all ionic compounds have a charge of 0.
2 - 2 = 0
Why are trigonal planar and trigonal pyramidal different in shape even though they both have 3 noncentral atoms?
Fill in the missing coefficient in the equation below.
__H2O2 → 2H2O + O2
2H2O2 → 2H2O + O2
In chemistry, "amu" stands for _______________.
In chemistry, "amu" stands for atomic mass unit.
How many particles are in 180.3 g of SiO2?
180.3 g SiO2
=
3 moles SiO2
=
1.806 x 1024 SiO2 molecules