These are the 2 particles found in the nucleus
Protons & neutrons
Negative
This is the term for the phase change from a solid to a liquid
Melting
As altitude increases, atmospheric pressure _____
Decreases
On the pH scale, what number is neutral?
7
An atom has an atomic number 10 and a mass of 24. How many neutrons does its nucleus contain?
14 neutrons
The S orbital holds ____ (#) electrons.
2
This is the shape of a molecule with the formula AB4
Tetrahedral
This is the term for a gas that is able to be pressed into a smaller volume
Compressible
An acid is a substance that donates _____ ions
Alpha decay
What type of wave has the lowest frequency?
Radio waves
This is the term for a bond in which electrons are shared unequally
Polar
This scientist's law states that P1V1=P2V2
Boyle
What is the term for a substance that can act as both an acid and a base?
Amphoteric
A substance has a half life of 10 minutes. If you begin with 40 g of the substance, how much will remain after half an hour?
5 g
Which part of the electromagnetic spectrum includes waves that can be used to detect patches of hot and cold?
Infrared
This is the term for the phase change that goes directly from a solid to a gas
Sublimation
If the volume of a container is doubled, what happens to the pressure within the container?
It is reduced by half.
What 2 things does a neutralization reaction produce?
Water and a salt
It beta decay, what happens to an atom's atomic mass?
It stays the same!
This is the term for the number of times a wave passes by in a given period of time.
Frequency
This is the ABE formula for this structure:
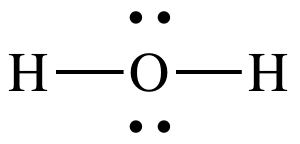
AB2E22
This is the conversion from Celsius to Kelvin
+273
What substance is the acid:
NH3 + H2O → NH4+ + OH-
H2O