List an element that will lose 1 electron to form a 1+ ion.
Li, Na, K, Rb, Cs, Fr
What is the percent composition of oxygen in H2O?
~89%
Write the following word AND balanced formula equation:
Solid sodium reacts with oxygen gas to produce solid sodium oxide.
2Na (s) + O2 (g) --> 2Na2O (s)
chromium (III) bromide
CrBr3
What year did Mr. Snyder graduate RV?
2017
Ionic Bond- formed between metal and nonmetal, high melting point, high conductivity
Covalent Bond- formed between nonmetals, low melting point, low conductivity
How many moles are in 117 g of Mg(OH)2?
2.01 mols
Classify the following equation in 2 ways:
Al(OH)3 (s) + H2SO4 (aq) --> Al2(SO4)3 (aq) + H2O (l)
Acid/Base & Double Replacement
K2CO3
potassium carbonate
Where is Mr. Snyder traveling to this summer?
Draw the Lewis Structure for the nitrite ion: NO21-
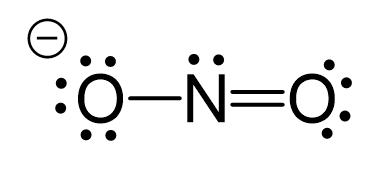
If I have an unknown quantity of gas at a pressure of 1.2 atm, a volume of 31 liters, and a temperature of 87ºC, how many moles of gas do I have?
1.26 moles
A students recovers 28 g of product when the theoretical yield is 40 g. What is the percent yield?
70%
N2O5
dinitrogen pentoxide
Where did Mr. Snyder recently get his masters from?
TCNJ
What are the 5 intermolecular forces?
London dispersion, Dipole-Dipole, Hydrogen bonding, Ion-dipole, Ionic bonding
Iron reacts with oxygen to form a compound that is 72.36% Fe and 27.64% O by mass. What is its empirical formula?
Fe3O4
Balance the following equation:
(NH4)3PO4 + Pb(NO3)4 --> Pb3(PO4)4 + NH4NO3
4(NH4)3PO4 + 3Pb(NO3)4 --> Pb3(PO4)4 + 12NH4NO3
CuCl
copper (I) chloride
Who was Mr. Snyder's chemistry teacher in high school?
Mrs. Gangel
What are the 5 basic molecular geometries? List the bonding:nonbonding ratios & bond angles for each.
Linear- 1:0, 2:0, 180º
Trigonal Planar- 3:0, 120º
Bent- 2:1 (<120º) or 2:2 (~105º)
Tetrahedral- 4:0, 109.5º
Trigonal Pyramid- 3:1 (~107º)
How much water must be added to 500 mL of 0.200 M HCl to produce a 0.150 M solution?
167 mL of water
Mg(OH)2 + 2HCl --> MgCl2 + 2H2O
If 2.5 g of Mg(OH)2 reacts with 750 mL of a 5.0 M HCl, how much MgCl2 is produced in grams?
4.1 grams
lead (IV) chromate
Pb(CrO4)2
What was Mr. Snyder's job in college?