What are the structural components of an atom and what are their charges?
Protons (positive charge) and neutrons (neutral charge) are located in the nucleus. Electrons (negative charge) are located around the nucleus in clouds.
How did Dalton transform Democritus's ideas on atoms into a scientific theory?
By using experimental methods
what happens when a photon hits an atom?
Electrons absorb energy
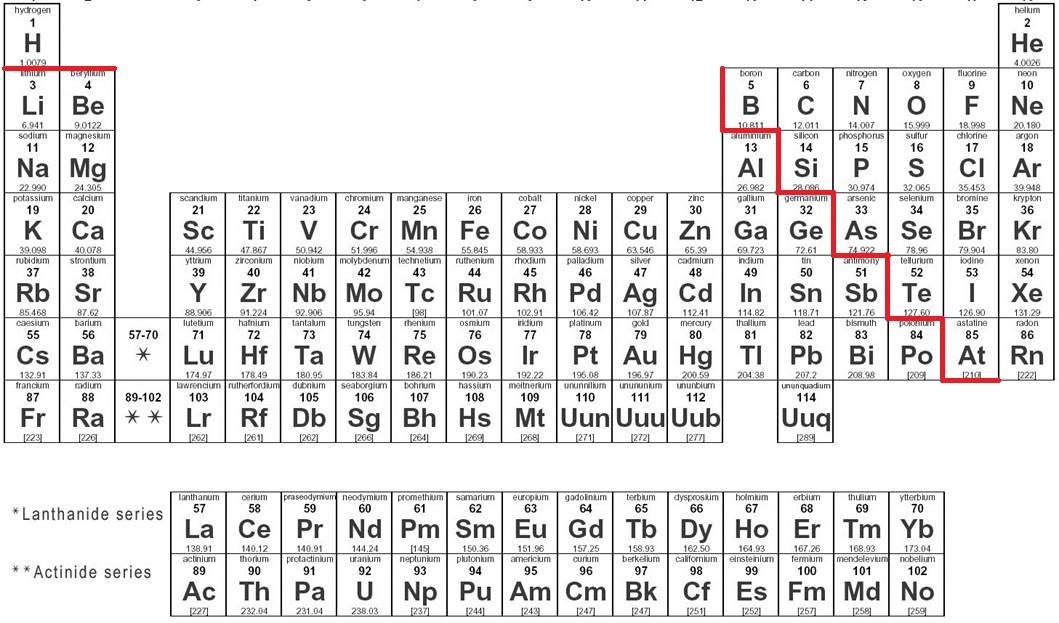 The "stairs" or "zigzag" on the table divides what?
The "stairs" or "zigzag" on the table divides what?
Metals and nonmetals
What is a universal, standardized form of measurement that is used by all scientists around the world?
The metric system.
Is most of the mass of an atom located in the nucleus or outside the nucleus?
Inside the nucleus because the mass of an atoms derives from the combined weight of its protons and neutrons which are located in the nucleus.
Who proposed the first atomic model and what was it called?
J.J Thomson and the Plum Pudding Model
They move up energy levels (away from nucleus).
What number tells you the number of protons and electrons?
Atomic Number
What are magnitude and dimension?
magnitude is the value of the number in the measurement and dimension is the unit of measure
What is the structural difference between an atom and an ion?
An ion has a different number of electrons than protons. This is the only structural difference.
What did Ernest Rutherford's Gold Foil Experiment conclude?
There is a positively charged nucleus in the middle of the atom.
Do shorter or longer wavelengths carry the most energy?
Shorter
What are columns on the periodic table called? How do they relate?
groups or families
they have similar chemical behavior
What is the unit of the mass of an atom?
Atomic Mass Unit (amu)
What does the term 'valence electrons' mean? (2 things)
number of electrons on outermost shell, tells how reactive an element is
What was the purpose of the Bohr's Model?
Showed the energy levels of an atom.
What happens when photons are released and the wavelength of the photons are within the visible spectra?
The material lights up (aka we see color)
What do the numbers mean that are around the element symbol?
Top left number is the mass number which shows the number of neutrons and protons.
Bottom left number is the atomic number which shows the number of protons in the element.
Top right number shows how many more protons than electrons the atom has. It shows the charge.
2.0 x 10^-3 has _ significant figures
2
^ the sig figs are 2 and 0
What is the mass of an electron, in atomic mass units?
approximately 0 amu
What does the Quantum Mechanical Model say about the energy of electrons?
As the energy of an electron increases its probability of being further away from nucleus increases.
Which color has the least amount of energy?
Red
^because it has the largest wavelength
Hydrogen, Helium, Neon, and Oxygen are all?
Nonmetals or gasses.
What are the base units for each type of measurement in the metric system?
Length = meter, m
Mass = gram, g
Time = second, s
Volume = liter, L
Temperature = kelvin, K