Neutrons have what kind of a charge?
Neutral/none
What does STP mean?
Standard Temperature and Pressure
The boiling point for a sample of a molecular compound is 80.° C at standard pressure. The heat of fusion Hf, of this compound is 127 joules per gram.
Determine the amount of heat required to completely melt a 50.0-gram sample of this molecular compound at its melting point.
6350J
What type of reaction produces salt and water as the only products in the reaction?
Neutralization
What is the chemical name for KClO2
Potassium chlorite
Where are electrons located?
Orbitals
What are the values of STP?
0C, 273K
101.3kpa, 1atm
What is the percent by mass of oxygen in CH3COOH (gram-formula mass = 60. g/mol)
53%
What are two types of organic reactions?
Answers must include two of the following:
Substitution
Fermentation
Saponification
Combustion
Addition
State, in terms of mass, why there is a large amount of heat produced during a nuclear fission reaction.
Mass is converted into energy/power
The subatomic particles in the nucleus of an oxygen atom include
neutrons and protons
Given the following:
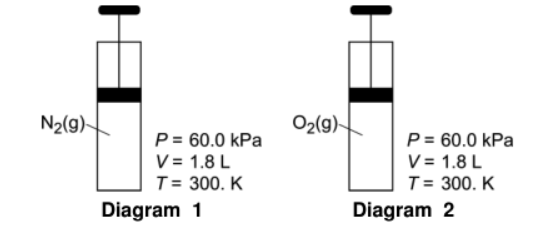
Compare the average kinetic energy of the gas in diagram 1 to the gas in diagram 2.
Equal
A NaoH Solution and an acid-base indicator are used to determine the molarity of an HCl(aq) solution. A 15.0 milliliter sample of the HCl(aq) is exactly neutralized by a 30.0mL of 0.010M NaOH(aq).
Using the titration data, determine the concentration of the HCl(aq) solution.
0.02M
State, in terms of carbon-carbon bonds, why octane is a saturated hydrocarbon.
All of the carbon-carbon bonds in octane are single bonds/all possible bonds are taken up by hydrogen.
Explain, in terms of elements, why the nuclear decay of U-234 to Th-230 is considered a transmutation.
A different element is being formed.
What is true about the charges and amount of protons and electrons?
They have opposite charges and are equal in number
OR
They have positive and negative charges; are equal in number
Given the following:
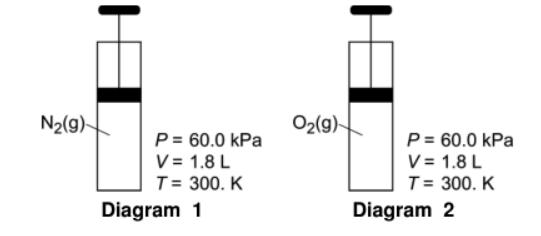
Determine the volume of the gas in diagram 2 when the conditions are changed to STP.
0.970-0.983L
A technician determined the percent natural abundance of Cu-65 in a sample to be 31.47%. Determine the percent error for this percent natural abundance of Cu-65 compared to the accepted value shown in the table.
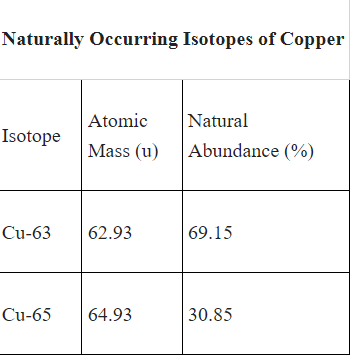
2.0-2.01%
State the number of hydrogen atoms in a molecule of hexane.
14
Oxidation occurs at the anode and reduction occurs at the cathode.
An atom of C-12 in the ground state and an atom of C-13 in the ground state are defined as isotopes of carbon because these atoms have the same number of protons and
Different numbers of neutrons
Given the following:
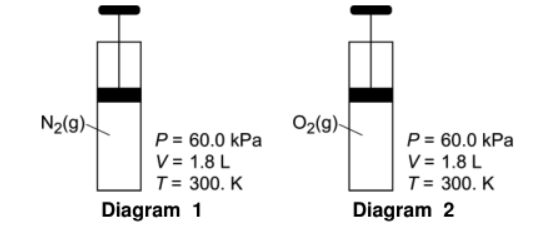
Determine the pressure given in diagram 1 in atmospheres.
0.59-0.6atm
Complete the nuclear equation for the decay of U-238 by writing a notation for the missing product.
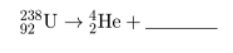
234
Th
90
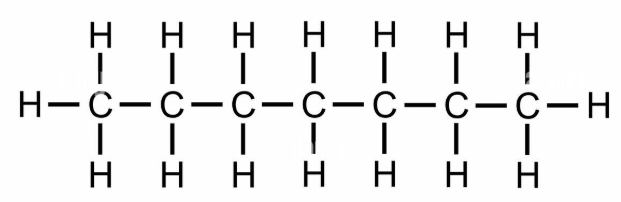
Draw a structural formula for a straight-chain molecule of heptane.
State how nuclear reactions such as fission, produce a large amount of energy/heat.
Mass is converted to energy.