In this type of bond, the electronegativity difference is so great that electrons are taken completely by one atom
What is ionic?
This is another general name for intermolecular forces, named after a person.
What are Van der Waals?
This type of reaction involves two elements or small compounds becoming one compound
What is synthesis?
__C3H8 + 5O2 --> 4H2O + 3CO2
This is the number that goes in the underlined spot
What is 1 / no number?
This is how many mL are in 0.034L
What is 34?
In this type of bond, the electronegativity of each atom is slightly different, causing electrons to be shared but on one side.
What is polar covalent?
If intermolecular forces increase, melting and boiling points will do this.
What is increase?
This type of reaction involves one element taking the place of another in a compound, and will only work in some cases.
What is single displacement?
__AlCl3 --> __Al + __Cl2
This is the number before Cl2 when balancedWhat is 3?
This is the scientific notation for 0.00000000000000270
What is 2.70 x 10-15?
In this type of bond, electronegativity difference is zero or near-zero, and electrons are shared fairly.
What is non-polar covalent
This type of intermolecular force exists between two polar molecules.
What is a dipole-dipole force?
In a neutralization reaction, water and this are always produced.
What is a salt?
This is the information gained when balancing an equation
What is the proportion of each substance involved (ratio of molecules)
This is the answer to (3.2x1034)(1.4672x10-30)
What is 4.7x104 or 47000?
The molecule CCl4's overall polarity. (Polar/nonpolar)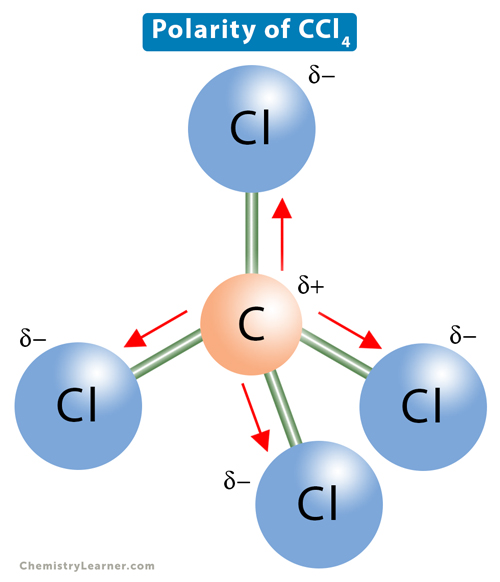
What is non-polar?
A bond between hydrogen and one of these THREE atoms will cause hydrogen bonding between molecules.
What are N, O and F?
Neutralization reactions between aqueous substances only happen if one product is this.
What is insoluble? (or a gas)
__C2H6 + __O2 --> __H2O + __CO2
This is the coefficient in front of O2 when balanced
What is 7?
This is the number of significant figures in 10.00020
What is 7?
The molecule H2O's overall polarity (polar/nonpolar)
What is polar?
This is the cause of London dispersion forces.
What is random movement of electrons, making non-polar atom/molecule temporarily polar?
A chemist performs a hydrocarbon combustion reaction and gets her face covered in soot. Which reactant would have an excess in this reaction?
What is the fuel? (CxHy)
This is the number of molecules of ZnCl2 produced when the following reaction is completed:
Zn + 2NaCl -->
What is ZERO? (Activity series!)
What is 24.5?