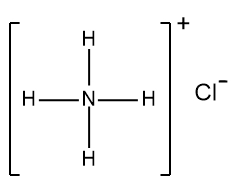
Write the dissociation reaction of HCl(aq)
HCl(aq) ---> H+(aq) + Cl-(aq)
OR
HCl(aq) ---> H3O+(aq) + Cl-(aq)
Rank the intermolecular forces from weakest to strongest
1. London Dispersion Forces
2. Dipole-Dipole Forces
3. Hydrogen Bonding
4. Ionic Bonding
What is the most electronegative element?
Fluorine (F)
------------------------------------------------
Electronegativity increases from left to right and bottom to top on the periodic table.
CaCl2(aq) ---> Ca2+(aq) + 2Cl-(aq)
According to the equation, how many moles of chlorine ion will be produced from 5.7 moles of CaCl2?
11 moles Cl-
---------------------------------------------------------
[5.7 mol CaCl2(aq)] x [(2 mol Cl-(aq))/(1 mol CaCl2)]
= 11.4 moles Cl- =~ 11 moles Cl-
_Al + _H2SO4 ---> _Al2(SO4)3 + _H2
Balance the equation
2Al + 3H2SO4 ---> 1Al2(SO4)3 + 6H2
(2, 3, 1, 6)
Sn2+ + 2Hg2+ ---> Hg2+ + Sn4+
According to the equation, which species is being oxidized?
Tin (Sn)
------------------------------------------------------
Oxidation is loss of electrons and gain of charge
Sn2+ -> Sn4+
Sn loses two electrons
What intermolecular force do all covalent compounds have?
London Dispersion Forces
What charge do group 2 elements have?
2+

Pb(NO3)2(aq) + NaCl(aq) ---> PbCl2(s) + NaNO3(aq)
According the the equation, how many moles of NaCl are needed to produce 350g of PbCl2?
1.26 mol NaCl
------------------------------------------------------------
[350g PbCl2] x [(1 mol PbCl2)/(278.1g PbCl2)] x [(1 mol NaCl)/(1 mol PbCl2)] =~ 1.26 mol NaCl
CuCl2(aq) + F2(g) ---> CuF2(aq) + Cl2(g)
Write the oxidation and reduction reactions for the given equation.
Oxidation: 2Cl-(aq) ---> Cl20(g) + 2e-
Reduction: 2e- + F20(g) ---> 2F-(aq)
--------------------------------------------------------
Cu2+(aq) + 2Cl-(aq) + F20(g) ---> Cu2+(aq) + 2F-(aq) + Cl20(g)
Cu2+ is a spectator ion
2Cl-(aq) ---> Cl20(g) + 2e-
2e- + F20(g) ---> 2F-(aq)
Write and balance the equation given the description:
Aluminium hydroxide and hydrochloric acid solutions produce aluminium chloride solution and liquid water.
Al(OH)3(aq) + 3HCl(aq) ---> AlCl3(aq) + 3H2O(l)
Identify all of the intermolecular forces in methane (CH4) and identify the strongest one.

London Dispersion Forces are the only and strongest forces in CH4
--------------------------------------------------------
Methane is a nonpolar molecule
What is the full electron configuration of phosphorus (P)?
1s22s22p63s23p3
Cu(s) + Ag2SO4(aq) ---> 2Ag(s) + CuSO4(aq)
According to the equation, how many grams of copper (Cu) are needed to produce 7.35 grams of silver (Ag)?
2.17g Cu
-------------------------------------------------------
[7.35g Ag] x [(1 mol Ag)]/(107.87g Ag)] x [(1 mol Cu)/(2 mol Ag)] x [(63.55g Cu)/(1 mol Cu)] =~2.17g
What is the molarity of a 100mL solution of 2.50M HCl if it is diluted to 500mL?
0.500M
------------------------------------------------
M1V1 = M2V2 ---> (M1V1)/(V2) = M2
[(2.50M)(100mL)]/(500mL) = 0.500M
Li(s) + NaCl(aq) ---> LiCl(aq) + Na(s)
Write the net ionic equation for the given equation.
Li0(s) + Na+(aq) ---> Li+(aq) + Na0(s)
--------------------------------------------------------
Li0(s) + Na+ + Cl-(aq) ---> Li+ + Cl-(aq) + Na0(s)
Cl- is a spectator ion
Identify all of the intermolecular forces in lactic acid (C3H6O3) and identify the strongest one.
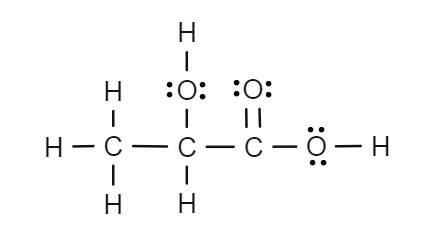
Hydrogen Bonding, Dipole-Dipole Forces, London Dispersion Forces. Hydrogen bonding is the strongest force.
-------------------------------------------------------
The molecule is polar and there is a bond between O and H
Which element does the following photoelectron spectrum represent?
Aluminium (Al)
1s22s22p63s23p6
C3H8(g) + 5O2(g) ---> 3CO2(g) + 4H2O(l)
According to the equation, how many mL of water will be produced from 21.70 moles of O2?
(the density of water is 1g/ml)
312.8mL H2O
------------------------------------------------------------
[21.70 mol O2] x [(4 mol H2O)/(5 mol O2)] x [(18.016g H2O)/(1 mol H2O)] x [(1mL H2O)/(1g H2O)] =~312.8mL H2O
What are two chemical names of H2O?
Dihydrogen Monoxide
Hydrogen Hydroxide (H-OH)
What is the difference between a strong and weak acid?
A strong acid completely dissociates. A weak acid partially dissociates.
-------------------------------------------------------
HNO3, a strong acid, will dissociate completely into H3O+ and NO3-
HNO2, a weak acid, will dissociate into H3O+ and NO2-, but leave behind some HNO2
Identify the intermolecular forces in ammonia chloride (NH4Cl) and identify the strongest one.
Ionic bonding is the only and strongest force in NH4Cl
-----------------------------------------------------
NH4+ and Cl- are ions
What is the valence electron configuration of antimony (Sb)?
5s25p3
AgNO3(aq) + NaCl(aq) ---> AgCl(s) + NaNO3(aq)
According the the equation, how many mL of 1.50M AgNO3 solution is needed to produce 5.00g of AgCl(s)
23.3mL
-----------------------------------------------------------
[5.00g AgCl] x [(1 mol AgCl)/(143.32g AgCl)] x [(1 mol AgNO3)/(1 mol AgCl)] x [(1000mL)/(1.50 mol AgNO3)] =~ 23.3mL
Calculate the pH of a solution with [OH-] = 5.32 x 10-4
pH =~ 10.70
--Solution 1----------------------------------
pOH = -log[OH-] = -log(5.32 x 10-4) = 3.27
14 - pOH = pH ---> 14 - 3.27 = 10.73
--Solution 2----------------------------------
1 x 10-14 = [H+][OH-] ---> 1 x 10-14 / [OH-] = [H+]
1 x 10-14 / 5.32 x 10-4 = [H+] = 1.89 x 10-11
pH = -log[H+] = -log(1.89 x 10-11) = 10.72