Arsenic (As) has ___ valence electrons.
Five
These two particles make up the nucleus of an atom.
Protons and Neutrons.
The Bohr Model represents this.
Atomic Structure.
What is the name of the circled number in this formula?
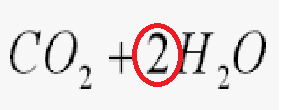
Coefficent
Is this a molecule or a compound molecule? 
Molecule
How many energy levels does Ca have?
4
This is the charge of a neutron.
Neutral.
What element is this?
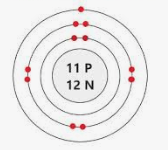
Sodium
What is the name of the circled number?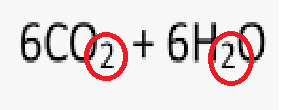
Subscript
Which two elements have the most similar chemical properties?
Phosphorus and Arsenic or Sulfur and Selenium.
An atom with two valence electrons will chemically bond with an atom with ____ valence electrons to stabilize its valence shell.
Six
This particle has a positive charge, while this one has a negative charge. (Two-part answer)
Proton and Electron.
You can tell how many protons and electrons make up an atom by knowing this.
The atomic number
How many atoms are in this chemical formula?
N2+3H2
8
Which side of the chemical equation is the reactant?
(1) (2)
2H2+O2-----> 2H2O
1
The maximum number of electrons that the Valence energy shell (outside energy level of an atom) can hold.
Eight.
This is how you find the number of neutrons in an atom.
Subtract the atomic mass from the atomic number.
The number of electrons that can occupy the first three energy levels of an atom.
2, 8, 18
Is this a balanced equation?
Al2+O3----> AlO3
No
Molecule or compound molecule?

Compound molecule.
The ______ valence electrons an atom has, the more chemically reactive the element is.
Less
These two atomic particles determine the mass of an atom.
Protons and neutrons.
What element is this:
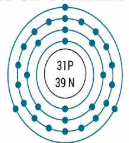
Gallium
How many atoms are in this formula?
2C4H6+7O2
34
Complete the sentence:
Every ______ is a molecule, but not every ______ is a compound.
Compound, molecule.