Draw the Bohr model of Hydrogen

Who developed the "Billiard Ball Model"?
John Dalton
Which elements are present in the compound H2O, and how many of each are there?
Two hydrogen atoms, one Oxygen atom
What are the charges of protons, neutrons, and electrons?
Protons = positive
Neutrons = neutral
Electrons = negative
What direction do the periods and groups of the periodic table go? I.e. which are rows (horizontal/left to right), and which are columns (vertical/up and down).
Periods are horizontal rows, groups are vertical columns.
How can you determine the number of energy shells (AKA levels/orbitals) of an element?
Shell number is equal to period number
What is the name of JJ Thompson's model of the atom?
Plum pudding/Raisin bun/Blueberry muffin model
Which of the following is correct?
1. All molecules are compounds
2. All compounds are molecules
2. All compounds are molecules
How can we determine the number of neutrons of an element, based on its element tile?
Number of neutrons = Atomic Mass - Atomic Number
What is the name of the family that is in group 2?
Alkali Earth Metals
Draw the Bohr model of Argon.
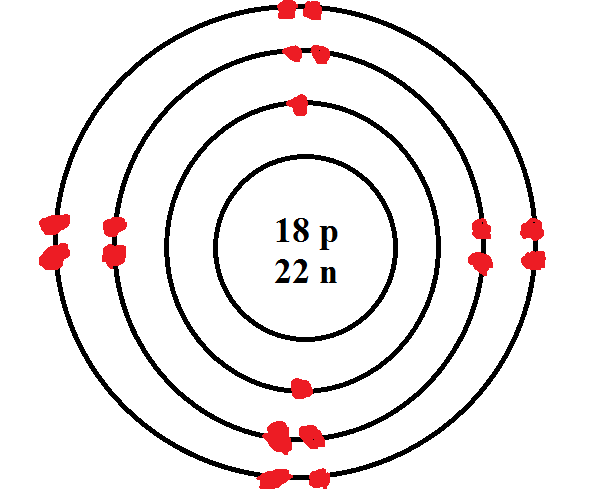
What is the name of the person who named the atom, "atomos"?
Democritus
How would you write the formula of a compounds that has one Sodium atom, one Hydrogen atom, one Carbon atom, and three Oxygen atoms?
NaHCO3 - this is the chemical formula for baking soda!
How many neutrons are in the element Titanium?
What are five pieces of information about an element (other than name and symbol) can you determine from the periodic table?
1. Number of protons
2. Number of neutrons
3. Atom's symbol,
4. Number of energy levels/shells
5. Number of valence electrons
What did Bohr compare his atomic model to?
He compared the Bohr model to the solar system, because the electrons orbit the nucleus like the planets orbit the sun.
Who completed the gold foil experiment, and what type of particle (including their charge) did he shoot at the gold foil?
Ernest Rutherford shot positively charged alpha particles at gold foil in his famous Gold Foil Experiment.
Which of the following molecules are compounds?
H2, CaCO3, H2O, Cl2, NaCl, Br2
CaCO3, H2O, NaCl
How many energy levels does Mercury have?
What are the names of the two families that make up the Rare Earth Metals?
Lanthanides and Actinides
What is the rule for where electrons are placed on the energy shells in a Bohr model?
Electrons are to be placed in the following order: North, East, South, West, first individually, then in pairs.
Your choice of food should represent a "positively charged" base (dough, pudding, etc.) with "negatively charged" pieces spread throughout.
Which aspect of an element determines how it will form compounds? (Hint: think about valence electrons)
Its reactivity
Why did Ernest Rutherford know that the nucleus is a positively charged mass, and that atoms are mostly empty space after completing the Gold Foil Experiment?
Because most of the positively charged alpha particles shot through the gold foil, but a few of them bounced off, Rutherford determined that the atoms of gold in the foil must have a positive centre that deflected the particles while those that did not hit the centre were able to pass through the foil.
Which two families of the periodic table react aggressively, and why? (Hint: think about valence electrons and reactivity)
Halogens and Alkali Metals react aggressively because Halogens (group 17) have seven valence electrons and Alkali Metals (group 1) have one valence electron. Because elements want a stable octet (eight valence electrons), Alkali Metals want to donate their one valence electron to a Halogen with seven valence electrons, so that each element has a stable octet.