Chemical or Physical
Atomic Math
Atomic Theory
Bohr Models
The Periodic Table
100
Clay is molded into a new shape.
Physical
100
How many protons are in Iron (Fe)?
26
100
Who was the first to develop an atomic theory?
Democritus
100

Carbon
100
What does the atomic number represent?
The number of Protons in the element.
That number also equals the number of electrons too.
200
/burninglogs-58dd324c5f9b584683b72ea0.jpg)
Chemical
200
How many neutrons are in Polonium with an atomic mass of 210?
126
200
What are two of the names of J.J. Thomson's model of the atom?
Plum Pudding
Chocolate Chip Cookie Dough
Raisin Bread
200
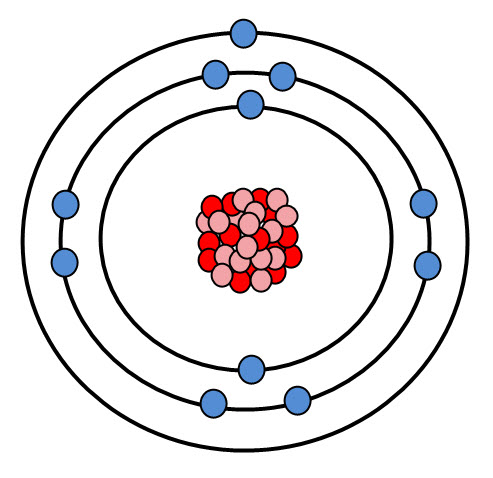
Sodium
200
How do you calculate the number of neutrons in an element?
Atomic mass - Atomic number = neutrons
300
Physical
300
What element will I have with an atomic mass of 137 and 81 neutrons?
Barium
300
This physicist's gold foil experiment lead the foundation of the current atomic theories, including the shape of the atom.
Ernest Rutherford
300
Draw the Bohr Model for Sulfur.

300
What is the name for the part of the periodic table that explains how many valence electrons are in an element?
Group or Group Number
400
Chemical
400
How many electrons will I have if my atomic mass is 131 and I have 77 neutrons?
***** Bonus Question *****
What element is that?
54
***** Bonus Answer *****
(worth 200 points)
400
This man developed the current atomic quantum model. His mathematical equation is more than 20 pages long to determine where an electron might be.
Schrodinger
400
Draw the Bohr Model for Bromine.

400
Which is a bigger atom:
Krypton
or
Cesium?
Cesium
500

Physical
500
If I have an element that has 104 protons and 157 neutrons, what would its atomic mass be? Which element would that be?
261
and
Rutherfordium
500
Put the scientists in order of the first to discover the model to the last:
Dalton
Thomson
Democritus
Chadwick
Rutherford
Schrodinger
Bohr
Democritus
Dalton
Thomson
Rutherford
Bohr
Chadwick
Schrodinger
500
Draw the Bohr Model for Argon.
+element..jpg)
500
What direction on the periodic table does reactivity decrease?
Left to right.