The difference in electronegativity between 2 bonded atoms
What is the way to find bond polarity?
8 electrons are needed to make an atom stable
What is the Octet Rule?
VSEPR shape
What do you look at to determine molecular polarity?
Valence, Shell, Electron, Pair, Repulsion
What does VSEPR stand for?
IMFs related to polar bonds
What are Hydrogen Bonding and Dipole-dipole forces?
Valence electrons are shared equally
What is a non-polar bond?
The three exceptions to the octet rule
What are Hydrogen, Beryllium, and Boron (think HEB)
These shapes always show molecular polarity
What do trigononal pyramidal and bent in terms of molecular polarity?
Symbolized as two dots on the central atom
What is a lone pair?
Non-polar related IMF
London dispersion forces
A bond with an electronegativity difference between 0.8 and 1.67
What is classified as a polar bond?
Carbon dioxide
What is the name for CO2?
Symmetrical VSEPR shape
What symmetry does a polar molecule have?

What is an example of an atom with a tetrahedral VSEPR shape?
The strength of IMFs in order (strongest to weakest)
Hydrogen Bonding, Dipole-dipole forces, London dispersion
A bond with an electronegativity difference of 1.68 or higher
What is classified as an ionic bond?
Ammonia
(write formula and give the other name)
Asymmetrical VSEPR shape
What symmetry does a non-polar molecule have?
5 main shapes (list them)
Linear, trigonal planar, tetrahedral, trigonal pyramidal, and bent
Where intermolecular forces are found/what they do
Found between neighboring molecules that hold molecules together
A pair of equal and oppositely charged poles, like that found in a polar bond
What is a dipole?

(give name and formula)
What is the Lewis Dot Structure for CCl4, or Carbon tetrachloride?

(what is its polarity)
What is an example of a non-polar molecule?
Water (shape)
(tip: draw Lewis Dot Structure)
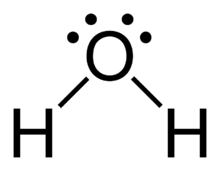
bent
Necessities for Hydrogen Bonding
Polar molecule with Hydrogen bonded with Nitrogen, Oxygen, or Fluorine