What does the atomic number tell us?
The number of protons in the nucleus.
Ionic bonds are formed through the ______ of electrons, while covalent bonds are formed through the ______ of electrons.
transfer, sharing
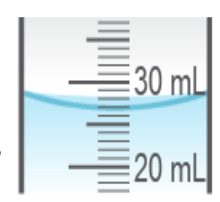 What is the correct measurement of the volume of liquid picturesd?
What is the correct measurement of the volume of liquid picturesd?
21.5
Write the formula for iron (II) sulfite
FeSO3
How many neutrons are in 14761Pm?
86
When heating a test tube, where should the opening of the test tube be pointed?
Away from all humans.
The mass number is equal to _____ + _____.
protons + neutrons
An atom becomes a negative ion by (gaining/losing) electrons.
The accepted temperature of a substance is 38 °C. The following data is collected in the lab:  This data (is/is not) precise; This data (is/is not) accurate.
This data (is/is not) precise; This data (is/is not) accurate.
The data is accurate but not precise.
What is the name of PCl3?
phosphorus trichloride
One atom has 20 protons, 19 neutrons, and 20 electrons. Another atom has 20 protons, 21 neutrons, and 20 electrons. Are these atoms isotopes or ions of the same element?
Isotopes
Rank the following four elements in order of increasing electronegativity: Be, C, Li, O
Li, Be, C, O
(Metals/nonmetals) are on the right side of the periodic table.
Metals
What is the molecular geometry of water?
bent
Calculate the density of substance C in g/L.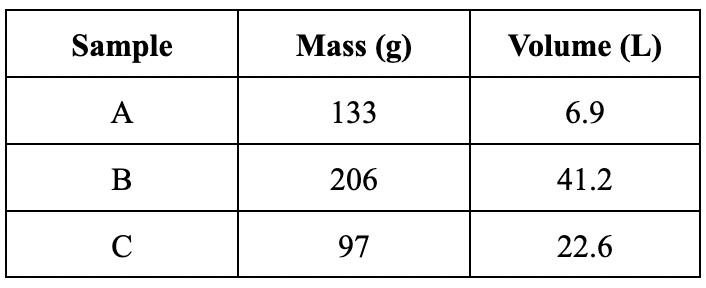
4.3 g/L
What is the name of NH4(NO3)?
ammonium nitrate
Boron has two isotopes. B-11 is 80.2% abundant. What is the abundance of the other isotope, B-10?
19.8%
Rank the following elements in order of increasing atomic radius: Si, Pb, C
C, Si, Pb
Elements A, B, and C have consecutive atomic numbers. If element C is a noble gas, what would the charge for an ion of element A be?
-2
Which of these is covalently bonded?
a) lithium phosphate
b) sulfur trioxide
c) sodium nitride
d) magnesium sulfate
b) sulfur trioxide
A beaker contained 36 mL of water; 5.2 mL was poured out, then another 14.44 mL was added. What is the final volume of water in the beaker? Be sure to report your answer to the correct number of significant figures.
45 mL
How many valence electrons does neutral phosphorus have?
5
Silicon has three naturally occurring isotopes. Use the table below to calculate the average atomic mass for silicon.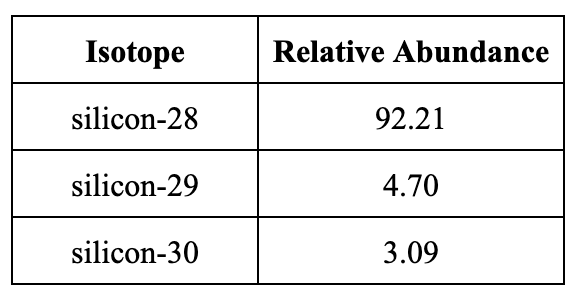
28.11 amu
Ionization energy follows the same trend as...
electronegativity
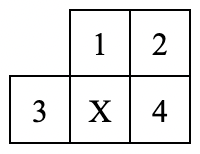 This diagram shows a small portion of the periodic table. Which element has one less proton than X?
This diagram shows a small portion of the periodic table. Which element has one less proton than X?
3
Which of these contains ionic and covalent bonds?
a) CO2
b) H2O
c) NaCl
d) Zn(NO3)2
d) Zn(NO3)2
How many significant figures are in the number 0.07301000?
7
When an atom becomes a positive ion, it becomes (larger/smaller).
smaller
A 600 g sample has a half life of 1.5 minutes. What mass of the sample remains after 4.5 minutes?
75 g
What is the molar mass of ZnCO3?
125.4 g/mol