What Scientist believed that all matter consisted of extremely small particles that could not be divided. He called them atoms from the Greek word atomos meaning "uncut".
Who is Democritus
What is this symbol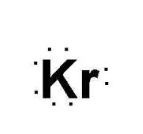
What is the Lewis Dot symbol
Which type of compound is comprises of a metal and non metal
What is an Ionic compounds
Name the reaction
A +B yields AB
What is synthesis
The formula for Density
What is Density equals Mass divided by volume
This Scientists model was that the atoms were tiny, indivisible, indestructible particles, and that each one had a certain mass, size, and chemical behavior that was determined by what kind of element they were.
Who is John Dalton (1766-1844)
Atoms react by gaining or losing electrons to fill the last energy level with 8 e- s, just like in Noble Gases. (Except He) for this rule
What is the Octet Rule
Name this compound MgO
What is magnesium oxide
This law established in 1789 by French Chemist Antoine Lavoisier that states that mass is neither created nor destroyed
What is Law of Conservation of Mass
Define Matter
What is anything that takes up space and has mass
Which scientist created the Plum Pudding model
Who is J.J Thomson
this type of bond occurs when there is complete transfer (between the two atoms) of the electrons in the bond.
What is an Ionic Bond
When naming compounds for which bond do you use Drop and Swap
What is the method for writing ionic chemical formulas
Name this reaction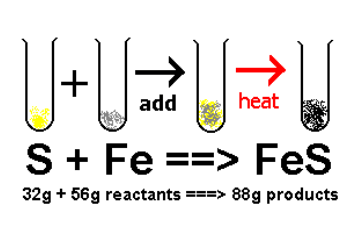
What is synthesis
What is this mnemonic device use for
King Henry Died By Drinking Chocolate Milk
What is a mnemonic device for the metric system
Which scientist completed Gold Foil Experiment.
Who is Ernest Rutherford (1871 – 1937)
Which bond is formed when a pair of electrons is shared between two atoms
What is a Covalent Bond
Write the chemical formula for
iron (II) chloride
What is FeCl2
Name this reaction
CH + O2
What is a combustion reaction
NaCl, Sodium Chloride is an example of for what type of matter
What is a compound
Which Scientist proposed the Planetary Model for the hydrogen atom in 1913. Electrons move in definite orbits around the nucleus, like planets. He proposed that each electron moves in a specific energy level.
Who is Niels Bohr
This element will never be a central element
What is Hydrogen
Name this compound
CuO
Copper II Oxide
Name this reaction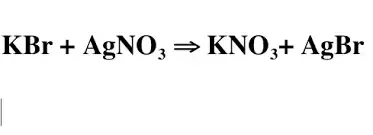
What is Double Replacement Reaction
Steel, Rain, Air are examples of this type of matter
What is examples of homogeneous mixtures