LiBr
What is LITHIUM BROMIDE?
Type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound
What are IONIC BONDS?
OF2
What is oxygen difluoride?
What atoms do in a covalent bond
What is SHARE ELECTRONS?
Symmetrical Molecules
What is NONPOLAR?
A solution that can conduct electricity
Aluminum Sulfide
What is Al2S3?
This type of element receives valence electrons in an ionic bond
What are NONMETALS?
Carbon Tetrachloride
What is CCl4?
Electron pairs to this to each other in covalent compounds, creating their shape.
What is REPEL?
Compounds with Hydrogen bonding
What is POLAR?
Polar solutes can only be dissolved in this type of solute
What is POLAR?
Silver Hydroxide
What is AgOH?
The overall charge in an ionic compound
What is NONE or NEUTRAL?
P4
What is tetraphosphide?
Name this shape.
What is TRIGONAL PYRIMIDAL?
Na-Cl
What is IONIC?
This status of solution where the solute completely dissolves in the solvent without heating.
What is UNSATURATED?
Ammonium Acetate
What is NH4C2H3O2?
 Give the chemical formula.
Give the chemical formula.
What is MgS?
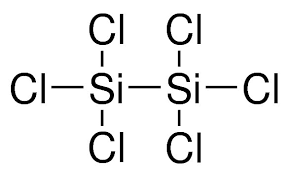
What is Disilicon Hexachloride?
The shape of a molecule that has 3 bonded pairs of electrons and no lone pairs.
What is trigonal planar or triagonal planar?
CO2
What is NONPOLAR?
What is SUPERSATURATED?
SnO2
What is Tin (IV) Oxide?
Another name for the charge of an atom
What is the OXIDATION NUMBER?

What is Diphosphorus pentoxide?
The total number of valence electrons Beryllium needs to be stable.
What are 4?
Compounds with London Dispersion Forces
What are POLAR, NONPOLAR, AND IONIC?
One of the following is a homogeneous mixture:
Salad, Ice Cream, Chicken Soup, Halloween Candy
What is ICE CREAM?