Sig Figs
Equal to 1000 base units
Kilo
How many sig figs?
456000
3
Calculate to the nearest sig fig
2.90 x 14.25
41.3
Unit of measurement for mass
grams
Element, Mixture, or Compound
Sodium
Element
What's shown in the image?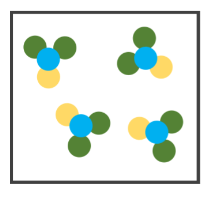
1 Compound
Deci
How many sig figs?
0.00008
1
Calculate to the nearest sig fig
7.050 + 6.2
13.3
Unit of measurements for volume
mL or cm3
Element, Mixture, or Compound
Water
Compound
What's shown in the image?
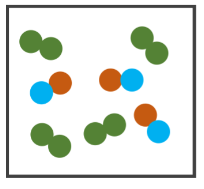
1 Compound and 1 Molecule (Element)
Equal to 0.001 base units
Milli
How many sig figs?
2.89 x 10-4
3
Calculate to the nearest sig fig
(2.03 x 104)(1.76 x 10-3)
3.57 x 101
Group of a single atom is called an...
Element
Which property?
Density
Physical
What's shown in the image?
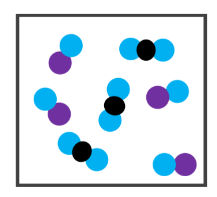
2 Compounds
How many km are in 1234 m
1.234 km
Convert 120000000 to scientific notation
1.2 x 108
Calculate to nearest sig fig
(1.901 x 10-7)(8.65 x 10-1)
1.64 x 10-7
Two or more elements chemically combined is called a...
Which change?
Oxygen produced from mixing calcium chloride and sodium bicarbonate.
Chemical
Rank the densities of these samples:
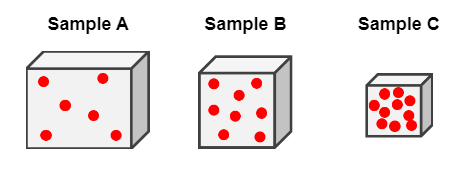
C > B > A
How many cm are in 50 m
5000 cm
Convert 2.89 x 10-4 to standard notation.
0.000289
Calculate to nearest sig fig
(9.11 x 103) / (5.4 x 101)
1.7 x 102
The ratio of an object's mass to it's volume is called...
density
Which change?
Ice melting
Physical
Rank the densities of the shavings in the graduated cylinders:
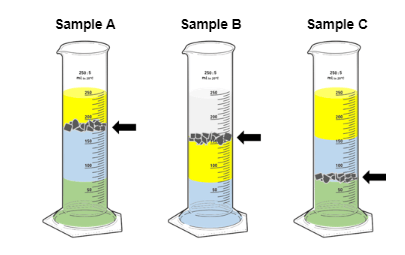
C > A > B