What is the atomic number of sulfur?

How many valence electrons do atoms want to have in their outer shell? (with the exception of hydrogen and helium)
8 valence electrons (hydrogen and helium want 2)
Which part of the atom is involved in making bonds with other atoms? Protons, neutrons, or electrons?
The electrons -- specifically the valence electrons.
If something is a solution or suspension, does that mean it is also a mixture?

Yes, solutions and suspensions are both types of mixtures. In solutions, the solute gets distributed evenly in the solvent. In suspensions, there are undissolved particles in water.

Is water polar or nonpolar? Does this mean electrons are shared equally or unequally?
Polar-- this means electrons are shared unequally

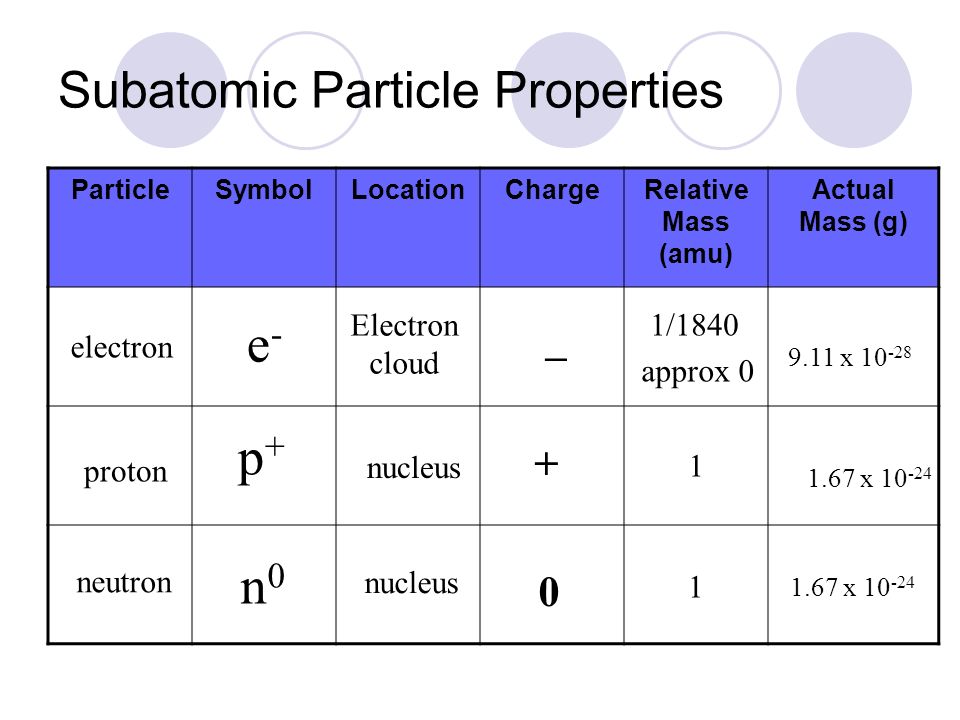
How many protons, neutrons, and electrons does neutral Aluminum have?

13 protons
14 neutrons
13 electrons

Electrons exist in energy levels around the nucleus of an atom. In the first three shells, how many electrons max go into each?
Shell #1: ______
Shell #2: ______
Shell #3: ______
Shell #1: 2 electrons
Shell #2: 8 electrons
Shell #3: 8 electrons
What type of bond would calcium (Ca) and sulfur (S) form? Would they be sharing or transferring electrons?
Ionic Bond because calcium is a metal and sulfur is a nonmetal. There would be a transfer of electrons.
What are the reactants and products in this chemical reaction formula?


What property of water describes the attraction of molecules of the same substance?
Cohesion

On the board, write out the symbols for protons, neutrons, and electrons.
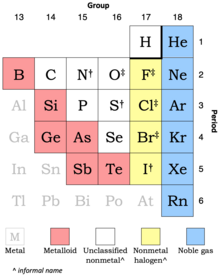
Based on what we know about valence shells, elements in which group # on the periodic table are LEAST likely to form bonds with any other atoms?
Group 18. Elements in this group all have full valence shells and thus will not share or transfer any electrons with any other atoms.
How many covalent bonds would carbon typically make?
4 bonds.
Because normally it has 4 valence electrons, it needs 4 more electrons to have a "full valence shell"
Why is water considered the universal solvent?
Because more substances dissolve in water than any other liquid.

What type of bond is stronger, an ionic bond or a Hydrogen Bond?
An ionic bond. Ionic bonds are formed by the mutual attraction of oppositely charged ions (atoms with + or - charges).
Hydrogen Bonds are weak bonds formed by the attraction of partially positive Hydrogens in a polar molecule to a partially negative atom in another molecule (in water, this atom is Oxygen)
What are the six most common elements in living things?
Sulfur, Phosphorous, Oxygen, Nitrogen, Carbon, Hydrogen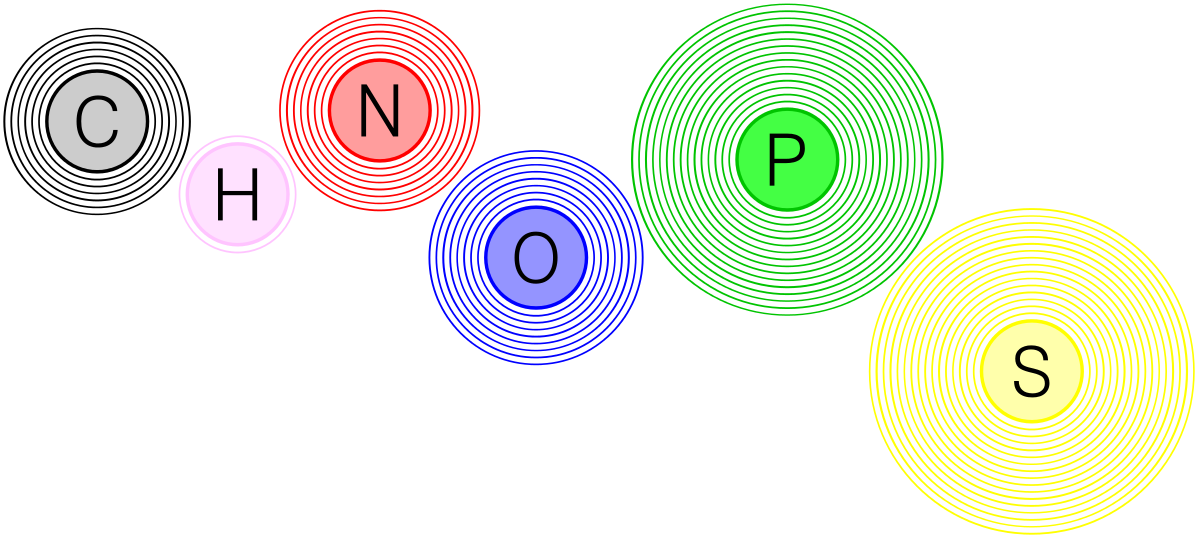
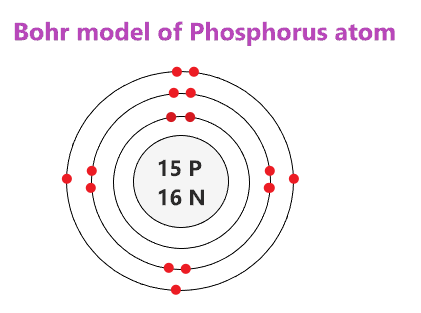
Draw the Bohr Model for Phosphorous.

In what type of bond are electrons shared equally?
A __________ _____________ bond
(must say both words)
Nonpolar covalent bond
Which type of substance is least likely to dissolve in water?
a. Ionic
b. Polar
c. Salts
d. Nonpolar
d. Nonpolar substances
"Like dissolves like"
Because water is polar, it has partial positive and negative charges and its molecules like to be around other charged or partially charged molecules.
It takes a significant amount of energy to increase the temperature of water. This is important for living things, which are mostly composed of water, because this property makes us more resistant to sudden changes in temperature. This property of water is called:
Specific Heat
Specific Heat Index
High Specific Heat

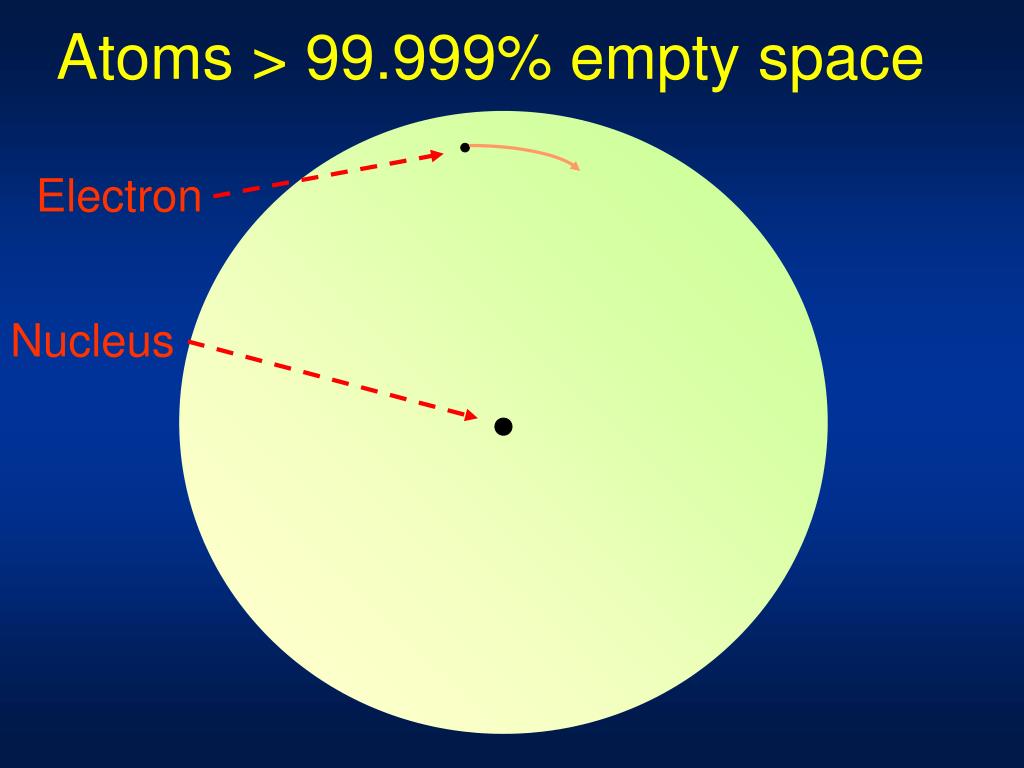
As we saw in the video of the beginning of the unit, in between the nucleus at the center of an atom, and the electrons moving in their shells, what lies in between?
Mostly empty space
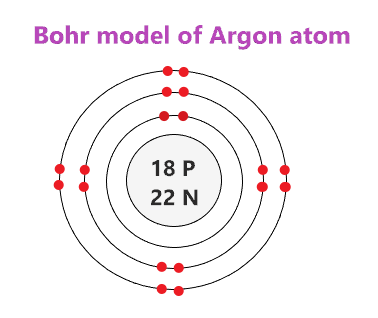
Draw the Bohr Model of an element that has a full valence shell. (multiple correct answers)
I will accept Helium, Neon, or Argon Bohr Models.
Draw the dot structure of CH2O.
I will draw it here:
In the chemical reaction shown, how many atoms of each element are present on the reactants side and products side? (# of C, H, and O on each side)

Reactants: 1 C, 4 H, 2 O
Products: 1 C, 2 H, 3 O

Draw two water molecules, show where their partial positive and partial negative charges are, and show the hydrogen bonding that occurs.