What are compounds and molecules both made out of?
Atoms
The thing that all compounds, Molecules, and extended structures have in common is that
They are all made of atoms that chemically combine
State of Matter with a definitive volume, but no definitive shape
Liquid (you need to know all 3 for the quiz)
What did the experiments in this activity demonstrate?
That when heated up, particles expand, but when cooled, particles slowed down.
What did the macaroni pieces represent in our ply atomic models? Why did every bonding site need a macaroni piece to be "legal"
the macaroni pieces represented chemical bonds.atoms must have all of their chemical bonds filled to combine with another and form a molecule.
What is this?
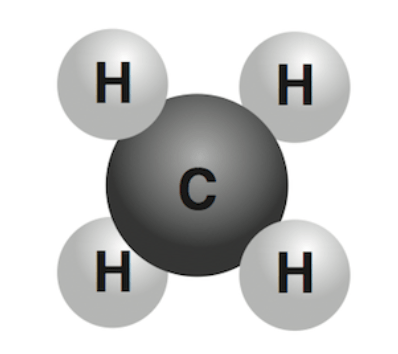
A Molecule and a compund.
Which State of matter has the lowest kinetic energy?
Solid has the lowest kinetic energy (you need to know how all three states of matter compare to each-other in terms of kinetic energy)
What are the three states of matter:
Solid, Liquid, Gas
If you were to sketch the molecule N2 the way we did in class, how would it look?
N≡N There are three lines because nitrogen bonds at 3 separate sites.
Is a Polymer made up of different types of molecules?
No. What makes something a polymer is the fact that it is the same exact molecule combined thousands of times.
Identify this state of matter:
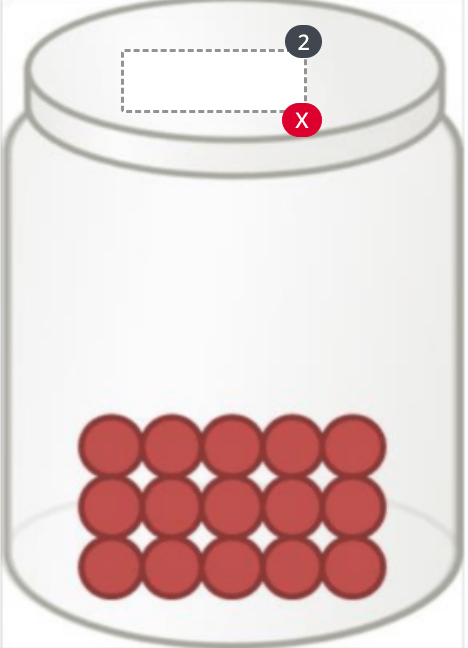
Solid (Know what the others look like as well)
At what temperature in Celsius, does water freeze and boil? What'a it's density?
Freezes at 0 degrees celsius
Boils at 100 degrees celsius
Density = 1.0 g/cm3
The Definition of particles is...
Small structures that make up all matter
What determines a substances properties?
it's chemical structure
Which State of Matter has no definitive shape or volume?
Gas
If a gas undergoes condensation and becomes a liquid, has it lost or gained kinetic energy?
It has lost kinetic energy (you need to understand which way kinetic energy increases or decreases based on state changes)
What's the difference between a molecule and a compound?
The only requirement to be a molecule is to have 2 atoms, chemically bonded. The requirement to be a compound is to have two different types of atoms be chemically bonded. This means that all compounds are molecules, but not all molecules are compounds.
(also you should be aware of what an extended structure is)
Look at the image below: Which molecule has the strongest bonds?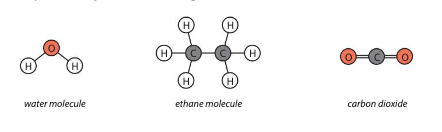
Carbon Dioxide because it is double bonded while the other molecules are only single bonded. This means it would require more energy to split them apart.
Is H2 a compound a molecule or both?
It is just a molecule because there are two atoms chemically combined, but there are not two different types of atoms chemically combined
What is the relationship between temperature and kinetic energy?
They correlate, as temperature increases, so does kinetic energy. As temperature decreases, so does kinetic energy.