A reaction in which 2 or more substances react to form a single substance.
Combination Reactions or Sythesis
Number of atoms in the following ion:
3O2
6 Oxygen Atoms
Is matter gained, lost, or conserved while balancing an equation.
Matter is conserved.
Predict the product of this equation:
Na + KBr -->
Na + KBr --> K + NaBr
A reaction in which oxygen reacts with another substance, often producing heat or light (CO2 and H2O).
Combustion Reaction
Ba + O
Ba2+ + O3-
Definition of a balanced equation.
When each side has the same # of atoms of each element and mass is conserved.
Predict the product and balance the equation:
PbSO4 + AgNO3 -->
PbSO4 + 2AgNO3 --> Pb(NO3)2 + Ag2 SO4
Is the following sample an acid or base based off these readings? What is the pH Range?
Methyl Orange- Red
Bromothymol Blue- Yellow
Phenolphthalein- Colourless
0-3 pH, Acid
A reaction in which a single compound is broken into simpler substances.
Decomposition Reaction
What are the charges of each atom and how many of each atom is there?
Mg3O2
3- Mg2+
2- O3-Balance the following equation:
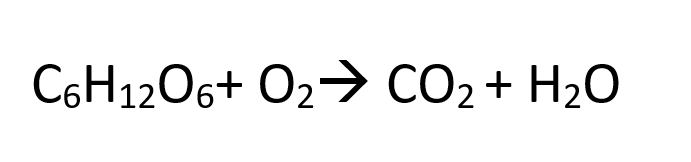
C6H12O6 + 9O2 --> 6CO2 + 6H20
Predict the product of the following equation:
KMnO4 + ZnCl2 -->
KCl + Zn(MnO4)2
Is the following sample an acid or base based off these readings? What is the pH Range?
Methyl Orange- Yellow
Bromothymol Blue-Blue
Phenolphthalein-Pink
10-14 pH
Base
A reaction in which the atoms of one element replace the atoms of a second element in a compound.
Single Replacement
How many oxygen atoms are on the product side of the equation?
C2H5OH + 3 O2 → 2 CO2 + 3 H2O
5
Balance the following equation:
Na3PO4+ MgCl2 --> NaCl + Mg3(PO4)2
2Na3PO4+ 3MgCl2 --> 6NaCl + Mg3(PO4)2
Predict the product of the following equation:
K + Cl -->
KCl
Is the following sample an acid or base based off these readings? What is the pH Range?
Methyl Orange- Yellow
Bromothymol Blue-Blue
Phenolphthalein- Colourless
7-9 pH
Base
A reaction that involves an exchange of positive ions between 2 compounds.
Double Replacement
How many individual Hydrogen atoms are in the entire equation:
3Ca(OH)2 + 2H3PO4 --> 6H2O + Ca3(PO4)2
24
Balance the following chemical equation
Ca3(PO4)2 + SiO2 → P4O10 + CaSiO3
2Ca3(PO4)2 + 6SiO2 → P4O10 + 6CaSiO3
Predict the product, balance the equation, and state the reaction type of the following equation:
PBr3-->
1. PBr3--> P3+ + Br1-
2. PBr3--> P + 3Br
3. Decomposition
Is the following sample an acid or base based off these readings? What is the pH Range?
Methyl Orange-Yellow
Bromothymol Blue- Yellow
Phenolphthalein- Colourless
pH 4-6
Acid