Define hypothesis as it relates to the scientific method.
A testable idea that explains a phenomenon.
What is the SI unit for mass?
Kilogram (kg).
What is the law of conservation of energy?
Energy cannot be created or destroyed, only transferred or transformed.
What is the charge and notation of a neutron?
Where do we go during a tornado drill?!
Hallway between main office & sanctuary
this term describes consistent patterns observed in nature.
What are the factors that affect precision in measurements?
Equipment
If melting point is a physical property, what is an example of a chemical property?
Reactivity, Flammability, Toxicity.
What distinguishes isotopes of the same element, affecting their atomic mass but not their chemical behavior?
Isotopes have the same number of protons but different numbers of neutrons.
Based on the hypothesis that the Bug-Block pesticide is harming the bee population, what differences would you expect to observe between treated and untreated fields?
A greater decline in bee numbers in treated fields.
Convert 132,547 millimeters to meters.
1000 millimeters = 1 meter
132.547 meters
Electrical energy is to _________ energy , as chemical energy is to _________ energy.
Electrical energy is to kinetic energy , as chemical energy is to potential energy.
What change to J.J. Thomson's plum pudding model was made after Ernest Rutherford observed that most alpha particles passed through the atom, but some were deflected at sharp angles?
The atom has a small, dense, positively charged nucleus, surrounded by mostly empty space.
In crowd crush at what density of people does a crowd switch from behaving like a gas to a liquid?
Around 5 people per sq meter.
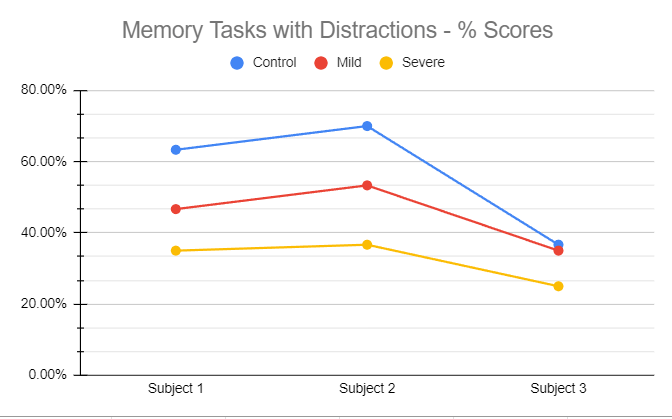
Order the subjects by their average performance, from highest to lowest.
1st : Subject 2
2nd : Subject 1
3rd : Subject 3
Calculate the percent error of an experiment where the theoretical weight of an element was calculated to be 32.3g but the experimental weight was found to be 34.7g.
7.43 %
In theatrical productions and chemistry labs, dry ice (solid carbon dioxide) is commonly used to produce fog or cool materials without leaving any liquid residue. This change of state is an example of what?
Sublimation.
Using a periodic table, what would be the combined mass of an Osmium, Francium and Gold atom.
*Use atomic mass.
Os : 190.23 amu
Fr : 223 amu
Au : 196.97 amu
610.2 amu
What phase changes would occur for a CO₂ sample maintained at 100 atm of pressure as the temperature is increased from -80°C to 0°C?
Solid to Liquid. Melting.
Uranium-235 is a commonly used element in nuclear reactors. How many protons and neutrons does Uranium-235 have?
*Use periodic table.
Protons : 92
Neutrons : 143
92 + 143 = 235
What has roots as nobody sees,
Is taller than trees,
Up, up it goes,
And yet never grows?
Mountains