What is the electron configuration for BERYLLIUM?
1s² 2s²
DOUBLE JEOPARDY!
What phrase is used to describe the delocalized electrons surrounding atoms in metallic alloys?
Sea of Electrons
NAME the following IONIC COMPOUND:
Be3N2
Beryllium Nitride
NAME the following COVALENT COMPOUND:
NO2
Nitrogen Dioxide
Define THE MOLE
The mole is a counting unit, in which 1 mole of any substances is equal to 6.022x1023 units.
DOUBLE JEOPARDY!
State the NAME and DESCRIPTION of the THREE RULES of AUFBAU ORBITAL DIAGRAMS.
AUFBAU'S RULE: Electrons must be placed in the lowest-energy orbital available.
HUND'S RULE: An electron must be placed in each orbital of a sublevel before being paired together.
PAULI'S EXCLUSION PRINCIPLE: Electron pairs must be represented with opposite spin.
DOUBLE JEOPARDY!
Describe the OCTET RULE.
Atoms gain, lose, or share valence electrons in order to have a complete-filled valence shell of 8 valence electrons.
NAME the following IONIC COMPOUND:
CuCl2
Copper (II) Chloride
NAME the following COVALENT COMPOUND:
P2O5
Diphosphorus Pentoxide
What term is used to describe the number of grams of any substance in 1 mole of that substance?
Molar Mass
EVERYBODY PLAYS! All teams can receive points for this question! After play, choose another question for the Toss-Up or Bonus.
Draw the complete AUFBAU Orbital Diagram for POTASSIUM!
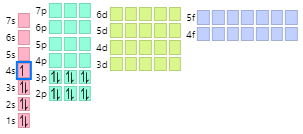
Examine the chart below:
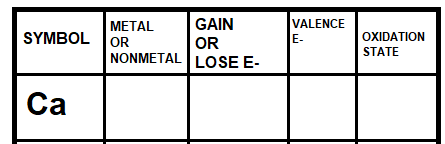 What information should be written in the blank boxes?
What information should be written in the blank boxes?
Metal, Lose, 2, +2
Write the CHEMICAL FORMULA for the following IONIC COMPOUND:
Sodium Nitrate
NaNO3
Write the CHEMICAL FORMULA for the following COVALENT COMPOUND:
CS
Carbon Monosulfide
QUADRUPLE JEOPARDY!
What is the molar mass of Copper (II) Phosphate?
380.58 g/mol
QUADRUPLE JEOPARDY!
What is the ELECTRON CONFIGURATION (Noble Gas Abbreviation) for SULFUR?
[Ne] 3s² 3p⁴
TRIPLE JEOPARDY!
Draw the Lewis Dot Structure for the bonding of MAGNESIUM and PHOSPHORUS.
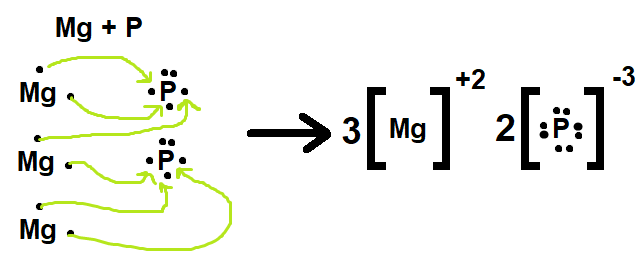
DOUBLE JEOPARDY!
NAME the following IONIC COMPOUND:
(NH4)2S
Ammonium Sulfide
DOUBLE JEOPARDY!
NAME the following COVALENT COMPOUND:
P5N7
Pentaphosphorus Heptanitride
How many atoms of Cesium are in 3.5 moles of Cesium?
2.1 x 1024 atoms
TRIPLE JEOPARDY!
What is the ELECTRON CONFIGURATION (Noble Gas Abbreviation) for Ti+2?
[Ar] 4s0 3d2
Titanium loses 2 electrons from its 4s valence shell.
Examine the chart below:
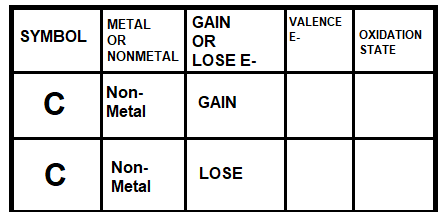
What information should be written in the blank boxes?
4 Valence Electrons, -4
4 Valence Electrons, +4
DOUBLE JEOPARDY!
Write the CHEMICAL FORMULA for the following IONIC COMPOUND:
Vanadium (V) Perchlorate
V(ClO4)5
DOUBLE JEOPARDY!
Describe the ERROR(S) with the following COVALENT compound's name:
Mononitrogen Decacopper
The prefix "Mono" is never used for the first element.
Copper is a metal, can not be listed second as an anion, and does not use covalent nomenclature.
QUADRUPLE JEOPARDY!
EVERYBODY PLAYS! All teams can receive points for this question! After play, choose another question for the Toss-Up or Bonus.
How many grams of Aluminum Oxide are in 7.5 x 1015 formula units of Aluminum Oxide?
1.3 x 10-8 g