The element found in all organic compounds
Carbon
in a voltaic cell the electrons flow through the _______ and the ions flow through the ____________
wire, salt bridge
Defined by a substance donating a proton
Bronsted-Lowry Acid
A reaction can occur when molecules collide with what two things?
Proper orientation and energy
What does this symbol represent?
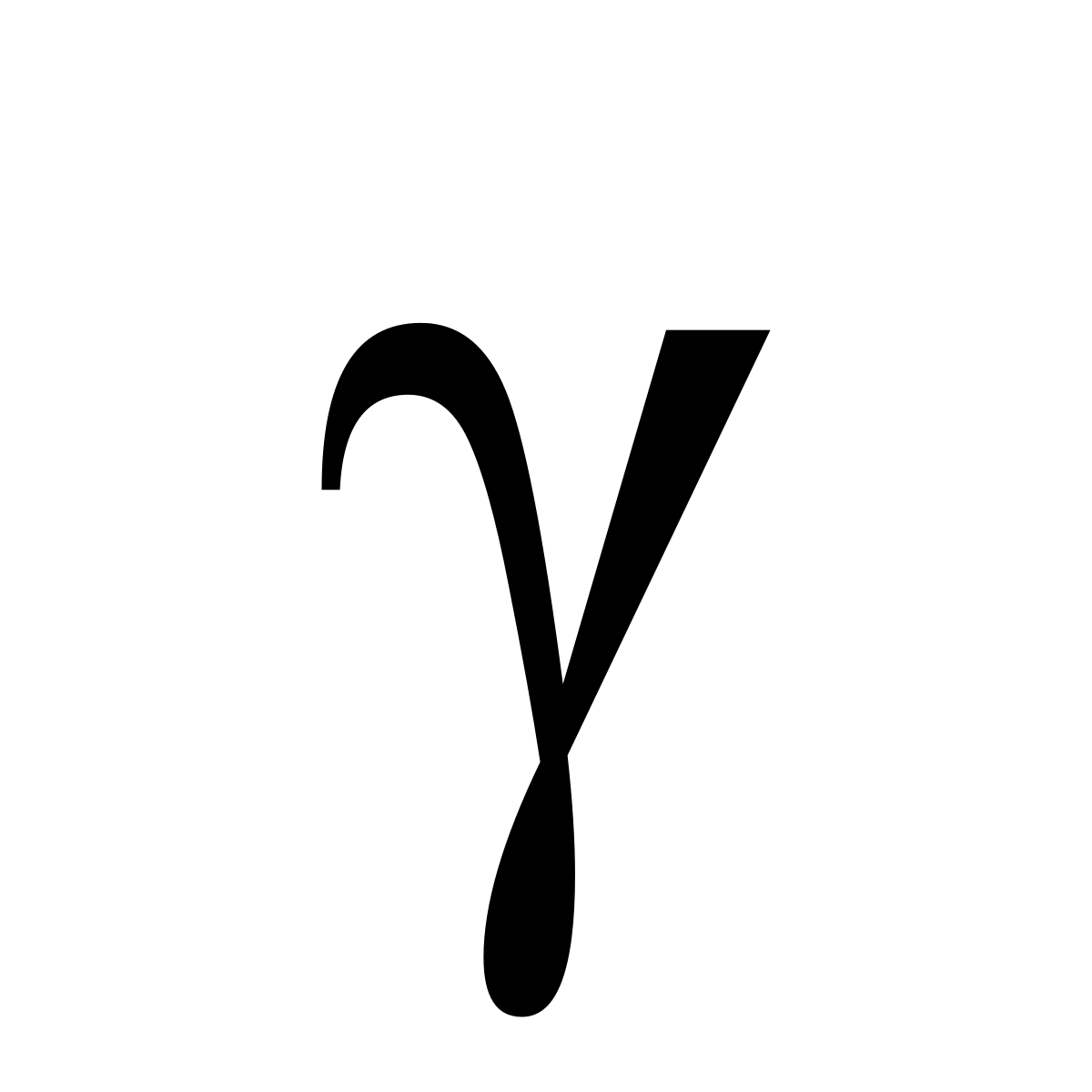
Gamma radiation
All isomers of octane have the same
Molecular formula
Part of voltaic cell that neutralizes the charges of each half cell
Salt bridge
What reaction between an acid and a base forms a salt and water?
Neutralization
List three things that would increase the rate of reaction.
Increase concentration, increase temperature, increase surface area
What isotope is used in geological dating?
U-238
A hydrocarbon has 7 carbons in a straight chain with a double bond between the 3rd and 4th carbon the name of the compound is
3-heptene
A salt bridge is filled with KCl. The K+ ions move to what half-cell?
The cathode
Which of these is not a base?
NaOH, KOH, CH3CH2OH, Ba(OH)2
CH3CH2OH
At 77 degrees celcius, which liquid on Table M has the lowest vapor pressure?
Ethanoic acid
How many half-lives go by for Fe-53 in 34.84 minutes?
4 half-lives
an unsaturated hydrocarbon + halogen -> halocarbon. This is an example of a(n) ____________
Addition ran
The electrode that gains mass in an electrolytic cell.
The cathode
A solution has a pH of 5.4. What color would the bromothymol blue indicator be?
Yellow
What is the activation energy, and is it endo or exothermic?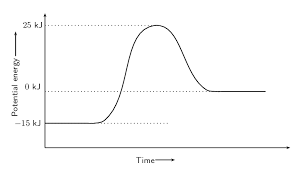
40 kJ, endothermic
How many reactants in an artificial transmutation vs natural transmutation?
2 vs 1
Name this compound.
4-methyl-3-hexanol
A cell where chemical energy is converted to electrical energy
Voltaic cell
A stock solution has a molarity of 4.7 M. How many mL of stock solution would you need to make 300 mL of solution with a molarity of 6.8 M?
434 mL
A + B --> C + heat
How would increasing the temperature affect the rate of the forward reaction?
Decrease the rate
What fraction of K-37 remains after 10.56 seconds?
1/8