- loses an electron, it becomes (-) charged
- gains an electron, it becomes (+) charged
False
True or False:
Temperature is based on kinetic energy.
True
True or False:
A molecule is any combination of two or more atoms.
True
What particles make up an atom?
a. protons
b. protons and neutrons
c. protons, neutrons, and electrons
C
What is an electron?
A subatomic particle that has a negative charge.
True or False:
An ionic bond occurs between a metal and non-metal.
True
What unit represents temperature?
a. STP
b. Kelvin
c. Celsius
d. AMU
Kelvin
True or False:
A compound is any combination of two or more different elements
True
What particles are located inside the nucleus?
Protons and neutrons
An electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond is known as?
Valence electron
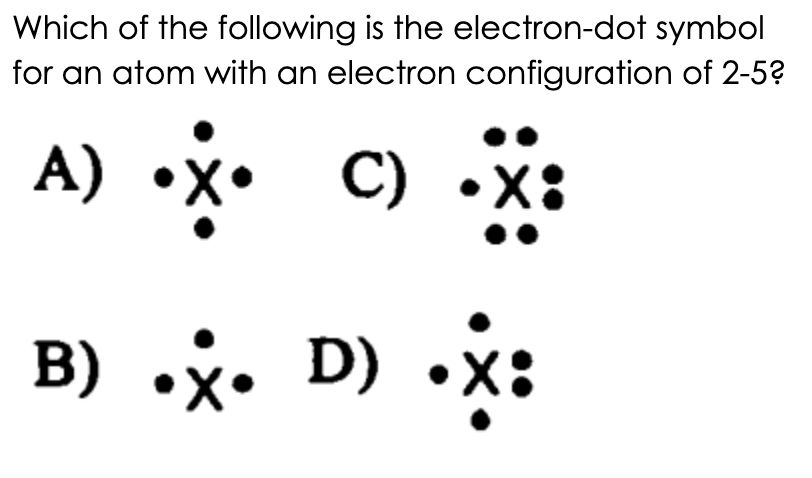
D
If the volume of the gas shrinks, what happens to pressure?
Increase
Is the picture below an example of a molecule or compound?
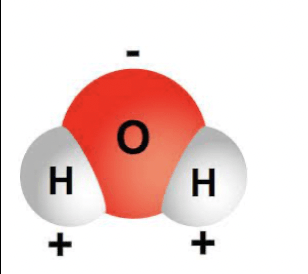
Compound
What is the atomic weight of the element below?

4.0026
What does the image below represent?
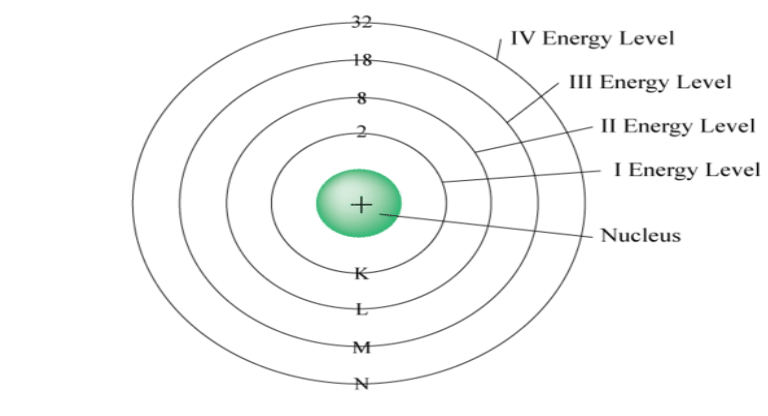
Bohr Model
True or False:
Covalent bonds form only between non-metals
True
If temperatures decrease, what happens to air pressure?
Decrease
True or False:
A mixture is when two or more things are combined together and chemically bond.
False
How many protons are in NA?
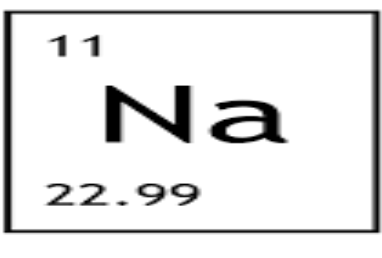
11
How many valence electrons are shown?
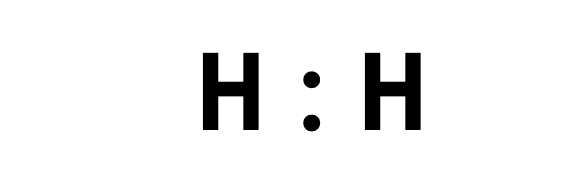
2
Is the image below a representation of an ionic or covalent bond?
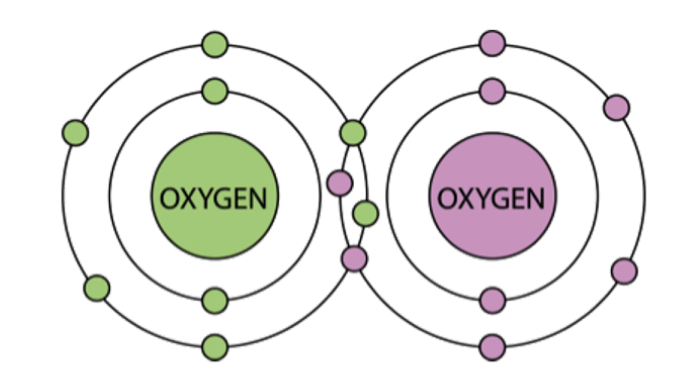
Covalent
When converting temperature from Celsius to Kelvin, how much do you add or subtract to Celsius?
273
What is the percent concentration formula?
What is the mathematical formula to find neutrons?
Neutrons = Mass number - atomic number
Describe the difference between a Bohr Model and Lewis Dot Structure model.
A Bohr model shows the number of electrons in each shell of an atom.
Lewis Dot structure shows the bonding between atoms of a molecule.