Has four properties that includes malleable, luster, conduct electricity and are ductile.
What are metals?
Any of the three phases in which an element can exist.
What is states of matter? Or What is solid, liquid, gas?
Horizontal rows on the periodic table.
What are periods?
When everyone is striving to be a noble gas.
What is the octet rule?
Atomic number of an element, has a positive charge.
What is a proton?
Simplest form of matter.
What are elements?
Has two characteristics of metals but not all four
What are metalloids?
The radius of an atom.
What is atomic radius?
Vertical columns on periodic table.
What are groups?
Energy required to REMOVE one electron from an atom of an element.
What is ionization energy?
Negatively charged subatomic particle, that can either be bound to the atom or free.
What is an electron?
The study of matter, the changes matter undergoes and the energry associated with those changes.
What is chemistry?
Not malleable, not ductile, has no luster and does not conduct electricity.
What are non metals?
Atoms or ions that have the same number of ELECTRONS.
What is isoelectronic?
Elements with similar properties; group 1, 2, 17 and 19
What are families?
A type of bond, when a halogen steals an electron from a metal?
Atomic mass - (minus) atomic number?
What are neutrons?
Located on the periodic table. Made up of either one better which is capitalize or two letter with the first capitalized and the second lower-case.
These are an example of it:
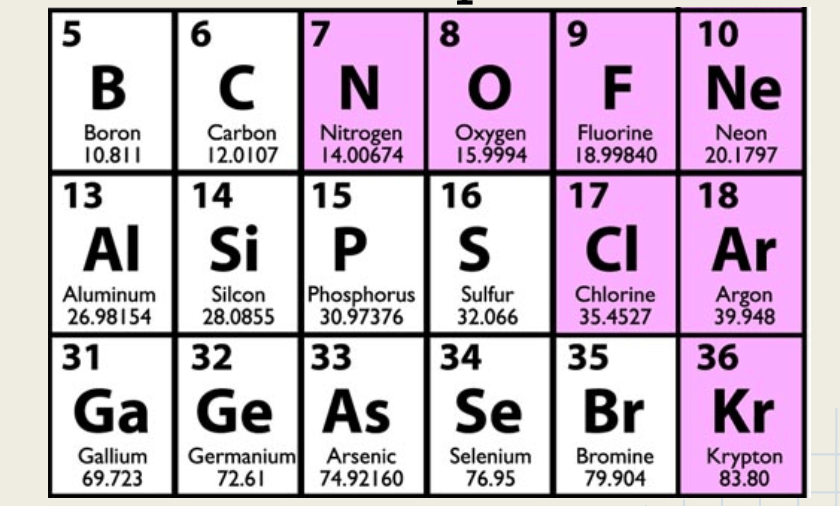
What are symbols? or element symbols?
Located along the "staircase" except for Aluminum (Al)
What are metalloids?
Which acid should come to mind?

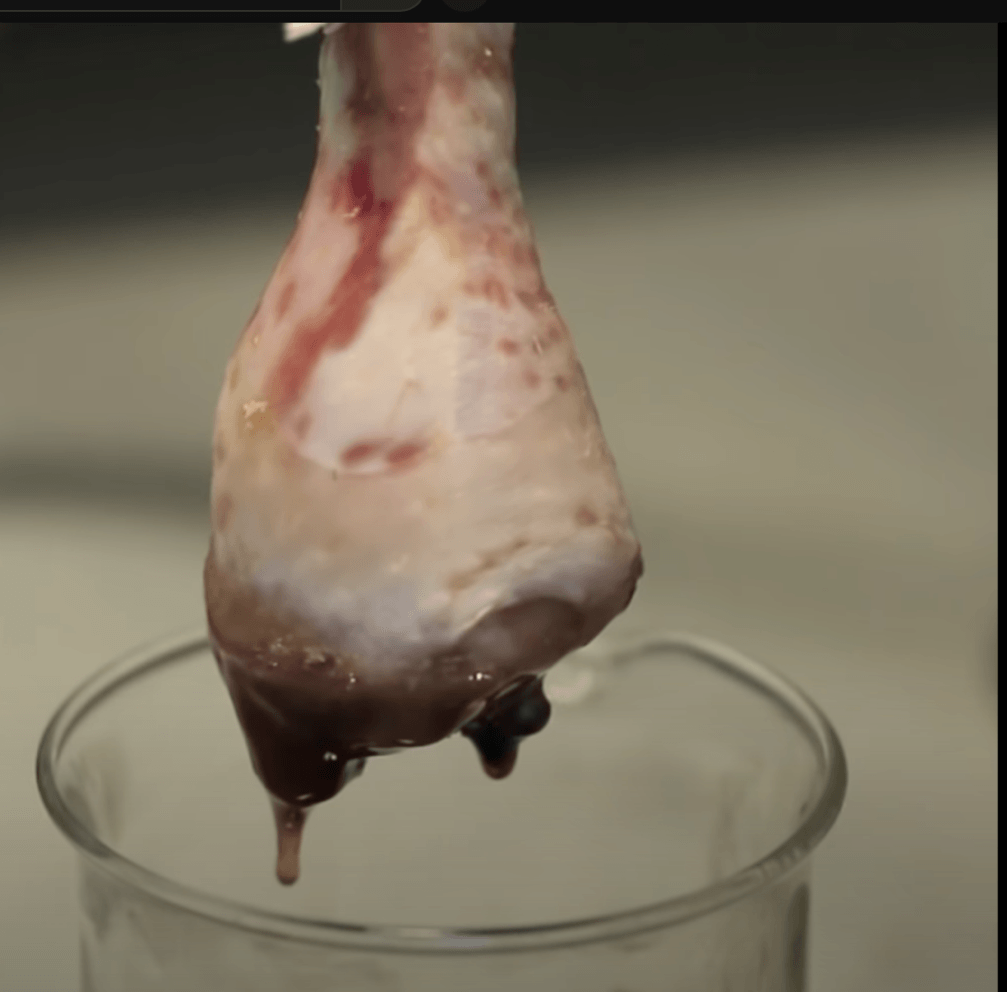
what is sulfuric acid? or hydrochloric acid? or hydrofluoric acid?
Reactivity is based on the elements with which they are combined
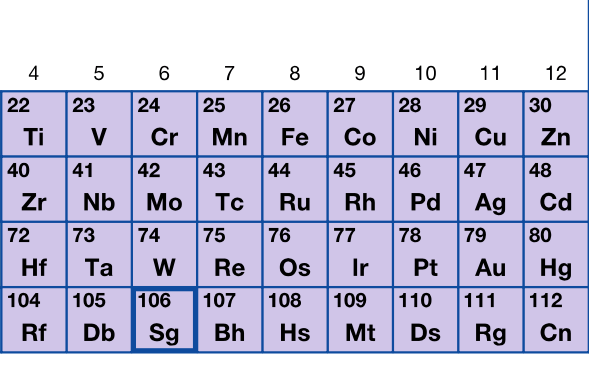
What are transition metals?
A type of bond, when someone in group 7 shares an electron with another member of group 7.
What is a covalent bond?
Each of these things have one (1) thing in common: 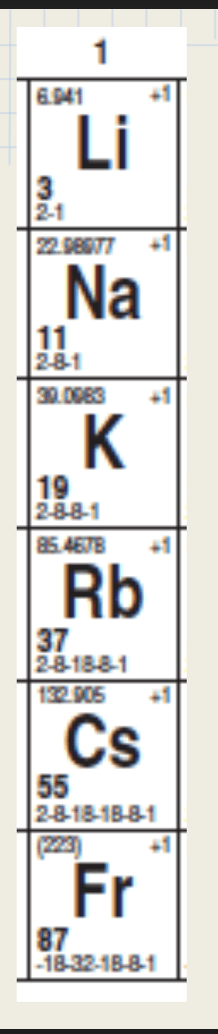
What is valence electron?
Consisting of two or more elements. Can only be changed by chemical means. H2O is an example of this.
What are compounds?
Located under/to the left of the staircase, except for Hydrogen (H).
What are metals?
As you move across a period, this happens.
Group 18 is this:
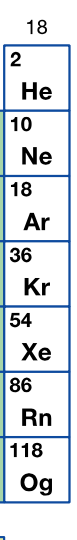
What are noble gases?
Metals lose electrons and become this.
What is a positive ion?
What do you call this?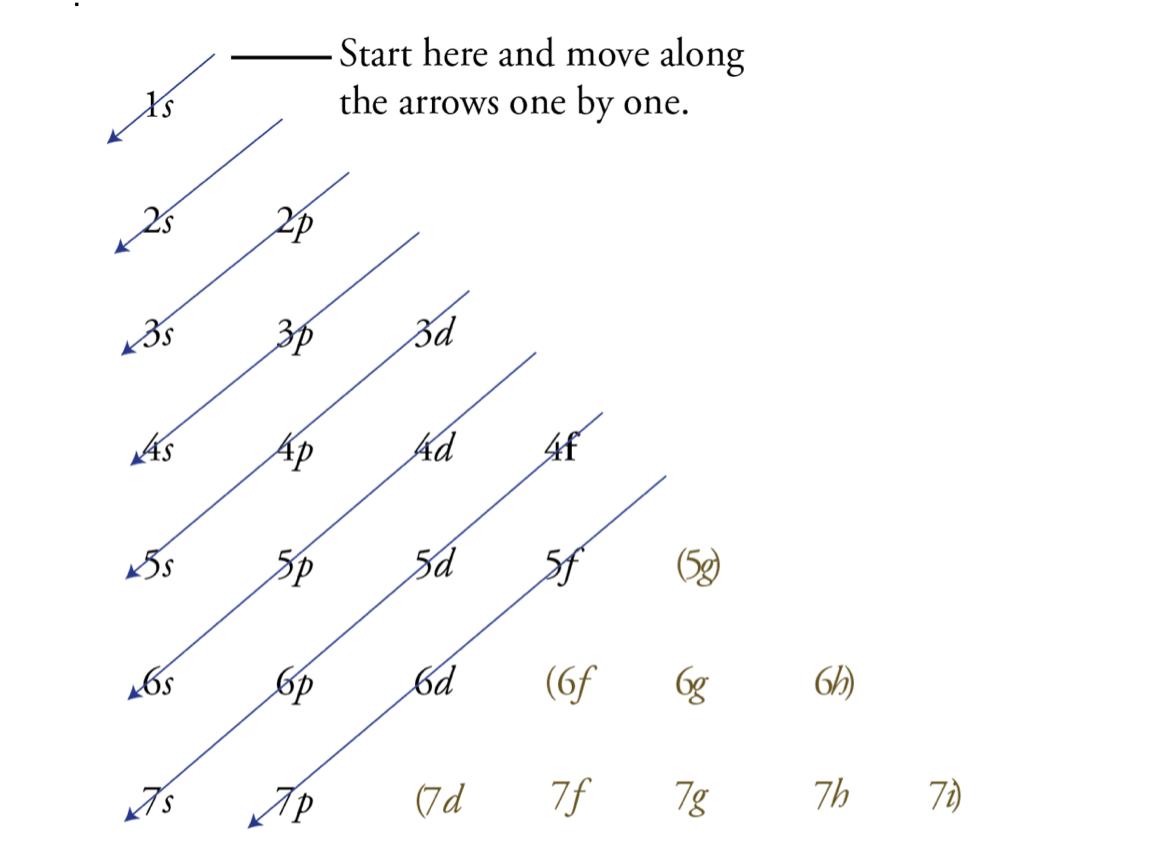
What is electron configuration or SPDF?
Surround the nucleus, electrons live here.
What is a shell?