What number tells you how many protons and electrons an element has?
The Atomic Number
Ice melting is an example of a physical or chemical change?
Physical
Protons have a _______ charge
positive
What do you write in the center of a Bohr Model?
The element symbol
A + B -> AB is what type of reaction?
Synthesis
How do you find the number of neutrons an element has?
You subtract the Atomic Number from the Mass Number
Physical Change
Electrons have a ______ charge
Negative
What do the dots in a Lewis Dot Structure represnet?
Valence Electrons
What type of reaction is represented by AB + C -> AC + B
Single Replacement
What do all elements in a group share?
The same amount of valence electrons
Type of change where a new substance is created
Chemical Change
What two subatomic particles create the mass of an atom?
protons and neutrons
The number of electrons that can fit in the 1st and 2nd orbital in a Bohr Model
2 and 8
What are the two products of a Combustion reaction?
Carbon dioxide and water
Why are Halogens (in group 17) so reactive?
They all have 7 electrons and only need one - high electronegativity
Name at least one sign that a chemical change has taken place
temperature change, bubbles created, or color change
Electrons have a ______ charge, so Anions ______ electrons and Cations _____ electrons.
negative, gain, lose
Draw the Lewis Dot Structure for Calcium

Coefficient
What period do you find noble gases AND why are they non-reactive?
Group 18 and because they have a full octet
An exothermic reaction releases energy or absorbs energy?
Releases
Where are protons found in the atom?
Nucleus
Name This Element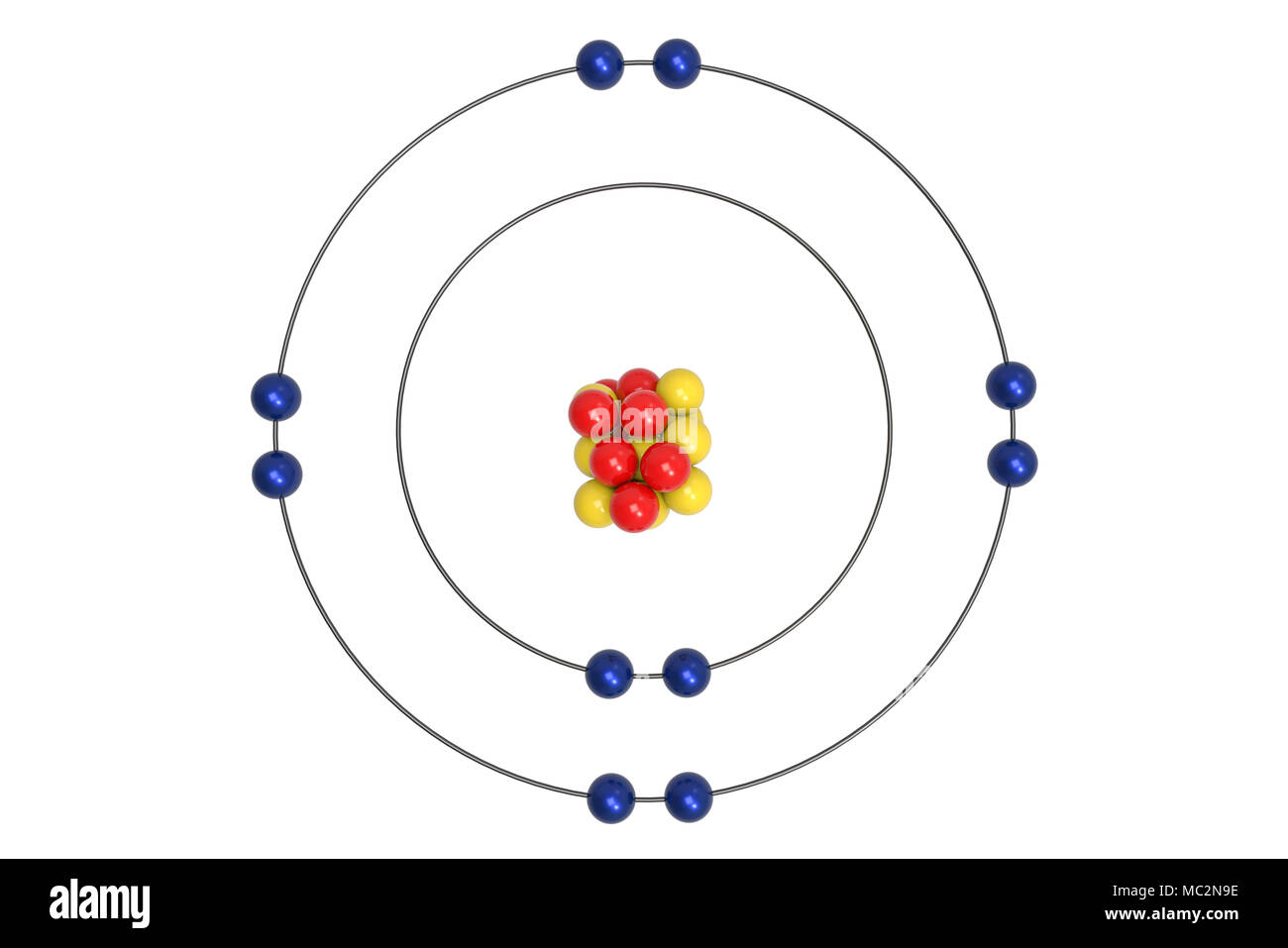
Neon
This equation: FeBr3 + 3 NaOH → Fe(OH)3 + 3NaBr is an example of what type of reaction?
Double Replacement