What is 10 moles of Na in grams?
230 g
What is the molar mass of: CaCOH
69.096 g/m
__ KClO3 --> __ KCl + __ O2
2 KClO3 --> 2 KCl + 3 O2
What is the law of conservation of matter?
Matter is neither created nor destroyed.
How many protons are in Si?
14
2 mols
What is the molar mass of: CuSO4
159.608 g/m
__ P + __ O2 --> __ P2O5
4 P + 5 O2 --> 2 P2O5
Which side of this equation is the reactants?
H2 + O ---> H2O
The left side.
How many valence electrons are in Arsenic?
5
How many moles in 5000 grams of K?
127.9 mol
What is the molar mass of: NaHCO3
84.006 g/m
__ C3H8 + __ O2 ---> __ CO2 + __ H2O
1C3H8 + 5O2 ---> 3 CO2 + 4H2O
The molar mass of an element is equal to its?
Atomic Mass
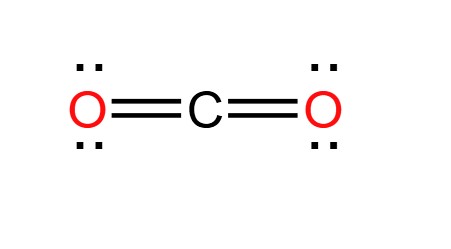
Draw the dot diagram for the compound CO2:
What is the mass in grams of 5.00 moles Ag?
539.5 g
What is the molar mass of: Cr4(P2O7)3
729.807 g/m
__ S8 + __ O2 --> __ SO3
1 S8 + 12 O2 --> 8 SO3
One mole of a substance is the same as its?
Molar Mass
Draw the electron cloud diagram for Argon:

How many molecules are in 3.00 moles of Li?
1.8 x 1024 molecules
What is the molar mass of: Co(C2H3O2)3
236.065 g/m
__ CO2 + __ H2O --> __ C6H12O6 + __ O2
6 CO2 + 6 H2O --> 1 C6H12O6 + 6 O2
A mole is ?
The mole is a unit of measurement that is the amount of a substance containing the same number of chemical units (atoms, molecules etc.) as there are Avogadro's number (6.022 X 1023).
Which element has a higher electronegativity Iron or Bromine?
Bromine