What is the maximum number of electrons that can go into the 5d sublevel?
10 electrons
What is a ionic bond?
It is the transfer of electrons between a metal and nonmetal. The electrostatic force of attraction that binds oppositely charged ions.
Balance the following reaction of benzene and hydrogen to form cyclhexane
C6H6 + H2--> C6H12
C6H6 + 3H2--> C6H12
What is the empirical formula of C6H12O2?
C3H6O
Arrange the following sublevels in order of increasing energy:
6p, 4d, 4p, 3s
3s, 4p, 4d, 6p
Is the following compound ionic or covalent?
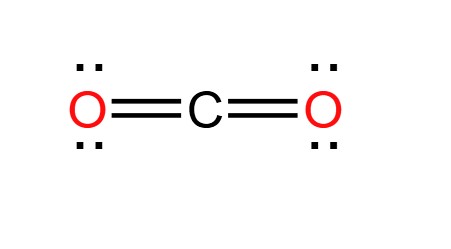
CO2
Covalent
Circle the letters of each cpmpound that can be produced by combustion reactions
a. Oxygen
b. Carbon dioxide
c. Water
d. Glucose
a. Oxygen
b. Carbon dioxide *
c. Water *
d. Glucose
What is the molar mass of acetic acid CH₃COOH?
60.0 g/mol
How many valence electrons does oxygen have?
6 valence electrons
What is the formula for the compound formed by the pairs of ions?
Fe2+ Cl-
FeCl2
Balance the equation of aluminum burning in oxygen to form aluminum oxide
Al + O2 -----> Al2O3
4 Al +3 O2 -----> 2 Al2O3
How many moles are in 9.03 x 1024 atoms of Hg?
15.0 mol Hg
What is the electron configuration of sulfur?
1s22s22p63s23p4
Why are nonmetals are likely to gain electrons than lose electrons?
Elements are most stable when their valence shell is completely full (octet).
Write a balanced synthesis reaction for Mg + O2
2Mg + O2 --> 2MgO
How many grams are in 14.4 mol F2?
547 g F2
What is the orbital diagram for Magnesium?

What is the electron pair geometry of CO2?
Linear
Fix the following incorrect reaction:
NH3 --> N + H3
2NH3 --> N2 + 3H2
How many grams are in 1 molecule of C9H8O4?
2.99 x 10-22 g C9H8O4