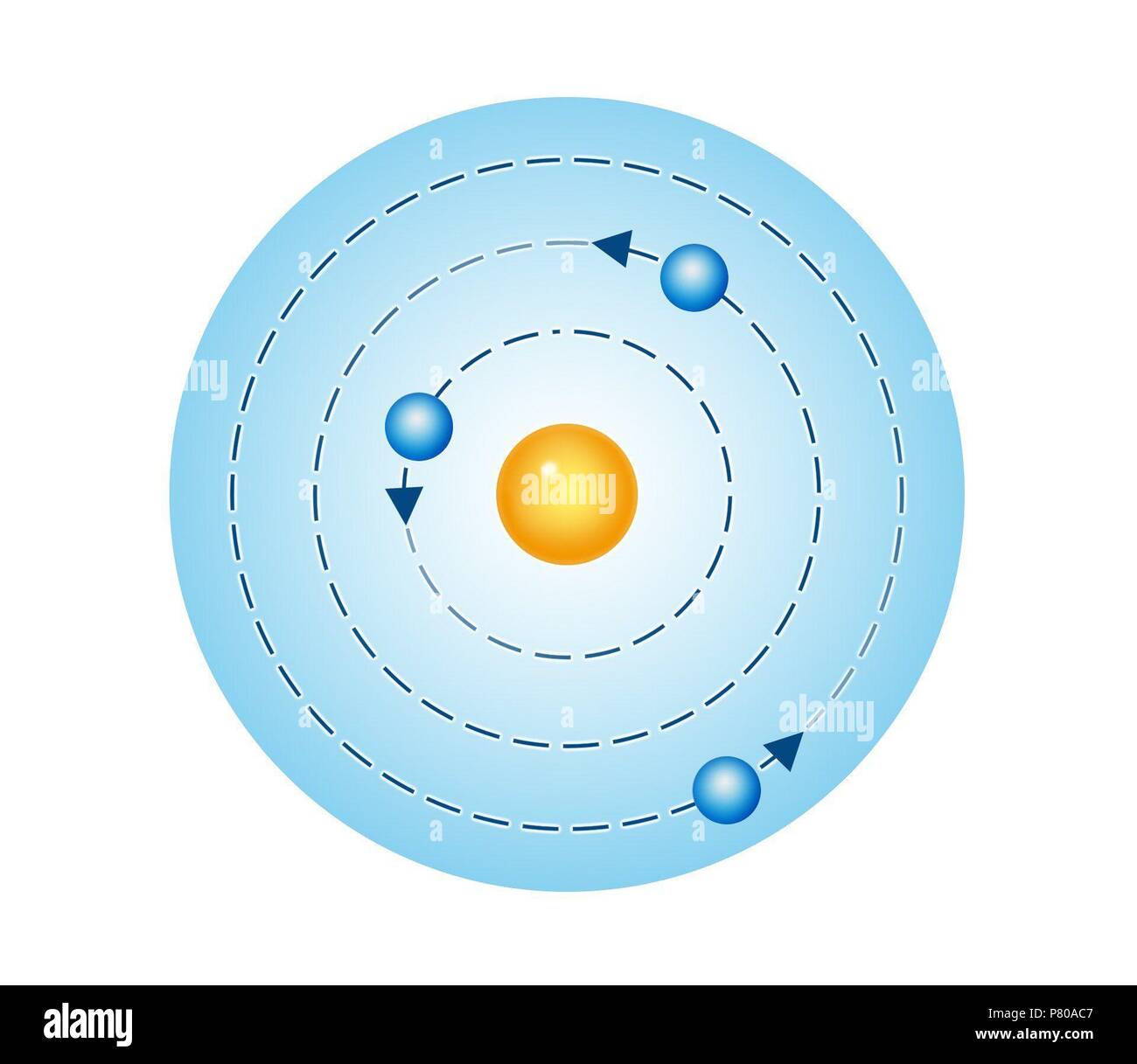
Bohr Model
Covalent
What particle is emitted from Alpha Decay?
He nucleus
What charge do protons have?
positive
What did Mendeleev do?
Periodic Table organization
This bond is between a metal and a nonmetal.
Ionic bond
Are the following measurements more ACCURATE or PRECISE when compared to the measurement of 100.0 degrees. Measurements: 77.4, 78.9, 77.1 degrees
PRECISE.
How many electrons does F have?
9
Name at least 1 of the 3 steps in the scientific method.
1) Making observations 2) Proposing and testing hypotheses 3) Developing theories
Identify the following as ionic, covalent, or metallic: MgO
Ionic
How many neutrons does C have?
6
What is the electron configuration of Oxygen? Write on the board.
1s22s22p4
What scientist was the first to prove the existence of an atom?
The Lewis Dot structure for aluminum would have ___ electrons?
3
What is the similarity and difference between an anion and a cation?
Both are ions where anions are negatively charged, and cations are positively charged.
How many valence electrons does Ne have?
8
Rutherford's Model, or Gold Foil Experiment
Identify the following compound as ionic, metallic, or covalent: CBrO2
Covalent
Three isotopes of oxygen occur in nature: oxygen-16, oxygen-17, and oxygen-18. If the atomic mass of oxygen is 15.9994, which of the three isotopes, if any, is the most abundant?
Oxygen-16
What early model of the atom did Thomson develop that involved a lump of positive charge with electrons stuck to it? Bonus Points: Draw model on board for double the points!
Plum-Pudding Model or
Blueberry muffin model
What is the charge for Na ion?
+1