The gold foil experiments of the early 1900's gives what three descriptions about the atom?
What is: Atoms have small, dense, positively charged nuclei.
Which formula contains a polyatomic ion?
K2S AlBr3 Al2O3 KCN
What is KCN
What is the empirical formula for hexane? C6H14
What is C3H7
In the following reversible reaction, water acts as an:
NH4 (aq) + OH2 (aq) Yields NH3(aq) + H2O(l)
What is an acid
What type of reaction is this?
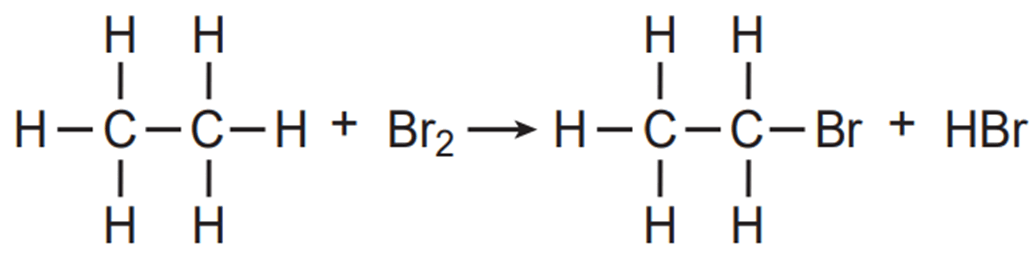
Single Replacement / Substitution
According to the electron cloud model, what term is the most probable location of an electron in an atom?
What is Orbital
What information about C4H10 can be determined from its structural formula, but not determined from its molecular formula?
What is arrangement of atoms
Calculate the formula mass of C9H11N2O2?
What is 163 g/mol
Which Molarity (M) solution is the best conductor of electricity?
What is highest concentration of ions
Which equation represents beta decay?
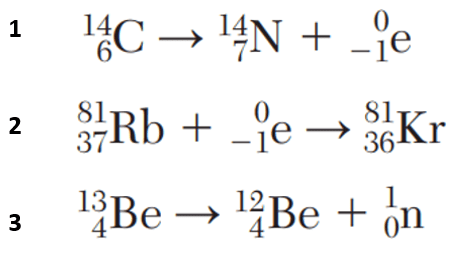
What is 1
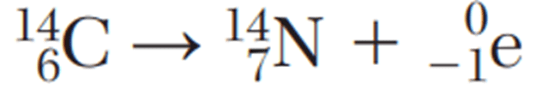
What change in energy occurs when an electron returns from a higher energy state to a lower energy state?
What is: energy is emitted.
What do the coefficients in a balanced chemical equation indicate about the substances in the equation?
What is mole ratios
Given a balanced equation representing a reaction:
2CO(g) + O2(g) → 2CO2(g) + energy
Which mass of O2(g) reacts completely with 5.6 grams of CO(g) to produce 8.8 grams of CO2(g)?
What is 3.2 g
Describe the heat transfer in this system:
A person with a body temperature of 40°C holds a cup of hot water in their hand. The temperature of the cup is 54°C. The air temperature is 2°C.
What is heat transfers from the cup to the hand to the air.
Nature favors an increase or decrease in energy and an increase or decrease in entropy?
What is the definition of an isotope of an element?
What is: same number of protons but a different number of neutrons
How many bond are there in N2?
What is triple bond
When 6.0 moles of electrons are gained by iodine, how many moles of electrons are lost by fluorine?
6KF + I6 → 6KI + F6
What is 6. Matter is not created nor destroyed, what is lost must be gained.
Which compound is an Arrhenius base?
NaCl Ca(OH)2 KNO3 H2SO4
What is Ca(OH)2
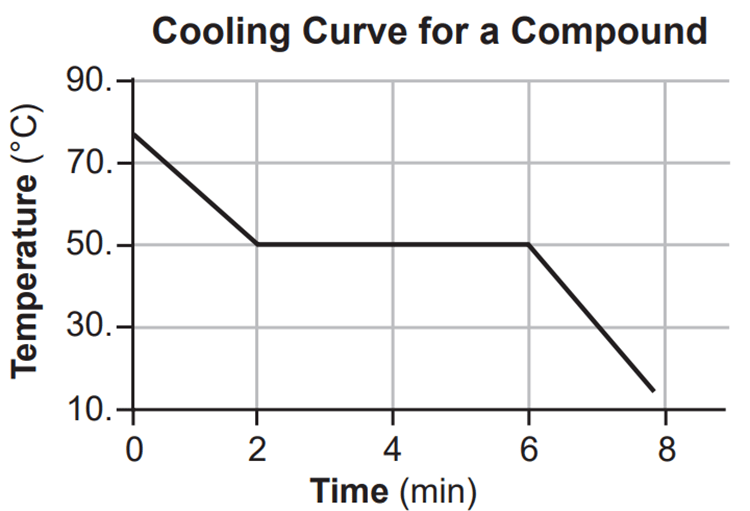
In terms of kinetic and potential energy what is happening with this cooling curve?
What type of nuclear reactions do these equations represent?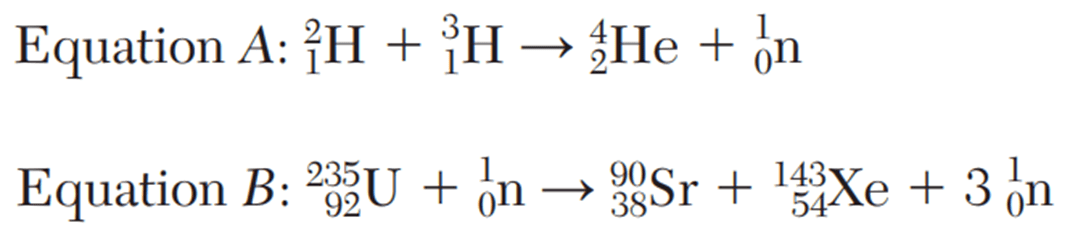
What is: Equation A is fusion, Equation B is fission
Name a super common molecule with an asymmetrical distribution of charge?
What is H2O
Forces of attraction between water molecules is known as?
What is hydrogen bonding
A solution has a mass of 5000 grams and contains 0.075 grams of dissolved solute.
What is the concentration in parts per million of this solution?
What is 15 ppm
Which phase change results in an increase in disorder?
Sublimination - solid to gas