What is an element?
A pure substance made of only one type of atom.
Which element has the symbol “O”?
Oxygen
What is cohesion?
Water sticking to itself.
What number is neutral on the pH scale?
7
What law states that matter cannot be created or destroyed?
Law of Conservation of Mass.
What type of mixture is evenly mixed throughout?
Homogeneous mixture.
How many hydrogen atoms are in H₂O?
2
What is adhesion?
Water sticking to other surfaces.
Is vinegar an acid or a base? (It has a low pH)
Acid
Reactants mass = 15 g. What must the products’ mass be?
15 g
Is trail mix a homogeneous or heterogeneous mixture? Explain.
Heterogeneous — you can see the different parts.
In a chemical reaction, what do we call the substances you start with?
Reactants
What property allows insects to walk on water?
Surface tension.
A substance has a pH of 12. What does that tell you?
It’s a strong base.
In a chemical equation, why must the number of atoms be equal on both sides?
Because atoms are conserved.
What is the difference between a compound and a mixture?
Compounds are chemically combined; mixtures are physically combined.
How many oxygen atoms are in C₆H₁₂O₆?
6
Droplets forming on a penny is caused by what two properties?
Cohesion and surface tension.
Why is water used as the reference point on the pH scale?
Water is neutral, with a pH of 7.
Explain how atoms change during a reaction.
They rearrange into new combinations but are not destroyed.
Classify each as element, compound, homogeneous mixture, or heterogeneous mixture:
Air, carbon dioxide, gold, pizza.
Air–homogeneous mixture, CO₂–compound, gold–element, pizza–heterogeneous mixture.
Why must chemical equations be balanced?
Because atoms are not created or destroyed — they only rearrange.
Explain how water moves up plant stems (capillary action).
Adhesion makes water stick to the stem; cohesion pulls more water upward.
Substance A has a pH of 9, substance B has a pH of 7.5, substance C has a pH of 2. Which substance is the acid?
Substance C
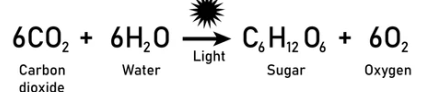 Use photosynthesis to explain conservation of mass
Use photosynthesis to explain conservation of mass
The atoms in CO₂ and H₂O rearrange to form glucose and oxygen; the total number of atoms stays the same.