The number of objects that makes 1 mole.
What 6.02 x 1023
The number of milliliters in 1 liter.
What is 1000
The formula of carbon tetrabromide.
What is CBr4
In a reversible reaction, this is the condition where the concentration of reactants and products remain constant.
What is equilibrium
Increasing the temperature of a gas that has constant volume causes this to happen to the pressure.
What is increases
The mass of 2 moles of C-14.
What is 28 grams
The number 0.0000062 converted to scientific notation.
What is 6.2 x 10-6
The name of the compound represented by the formula K2S
What is potassium sulfide
This coefficient would balance this equation if placed in front of H2O
What is 3
When you decrease the volume of a container of gas, this will happen to the pressure if the temperature is constant.
What is increases
These numbers can represent the number of atoms/molecules or the number of moles of a species in a chemical equation.
What are coefficients
The result when you convert 3 moles of helium to liters at STP
What is 67.2 liters.
The name of the compound NaNO3
What is sodium nitrate
The shift in equilibrium if NH3 is removed in the following reaction.
3 H2(g) + N2(g) <--> 2 NH3(g)
What is right (or forward)
What are increase pressure and decrease temperature.
 In this equation, it's the number of moles of carbon needed to react completely with 6 moles of iron III oxide (Fe2O3)
In this equation, it's the number of moles of carbon needed to react completely with 6 moles of iron III oxide (Fe2O3)
What is 9
This term describes a number, expressed in grams per mole, used to convert grams of a substance into moles.
What is molar mass
The formula for iron III sulfate
What is Fe2(SO4)3
How the equilibrium will shift if the temperature is increased in the following reaction.
2 HCl(aq) + Mg(s) <--> MgCl2(aq) + H2(g) + heat
What is to the left (toward reactants)
The shift in equilibrium caused by decreasing the gas pressure in the following reaction.
3 H2(g) + N2(g) <--> 2 NH3(g)
What is left (or reverse, backwards, toward the reactants)
The number of moles of Tetraphosphorus Decoxide (P4O10) produced by 1 mole of oxygen (O2) according to this equation.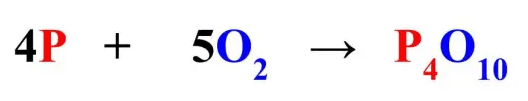
What is 0.2 moles.
What is 288 kelvins
The name of the compound represented by this formula: CuO
What is copper II oxide (not copper oxide)
Bohr discovered that frequencies of light missing in spectra were equal to specific amounts of energy absorbed by electrons. These amounts of energy are called this which inspired the name of a new field of physics.
What are quanta
The missing term in this problem using the combined gas law.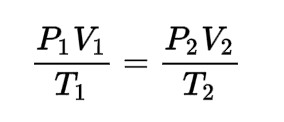
500.0 liters of a gas in a flexible-walled container are prepared at 700.0 mmHg and 200.0 °C. The gas is placed into a tank under high pressure. When the tank cools to 20.0 °C, the pressure of the gas is 30.0 atm.
What is V2