COUNT THE ATOMS
pure substances,
and compounds
properties / changes
The nucleus
the atomic number tells you the number of ___________
protons
H2O
3
what state of matter is this?
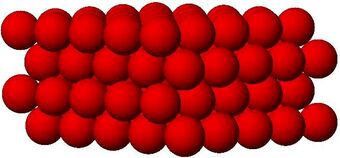
Solid
Define pure substance
all the atoms or molecules are the same throughout.
color
physical property
List 2 lab rules
no closed-toed shoes
no loose clothing
lab materials may not leave the classroom
do not put chemicals back in the conttainer
follow teacher's directions
no horseplaying
what are the charges of the 3 different subatomic particles
proton - Positive
Electron - Negative
Neutron - Neutral
to get the number of neutrons you subtract
____________ - ______________
mass - the atomic number.
5NaCl
10
what state of matter is this
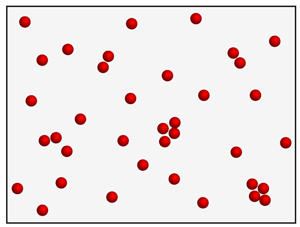
gas
is this a mixture, pure substance?

mixture.
taste
physical property
What is a hypothesis
an educated guess
What subatomic particle determines what element an atom is?
A proton.
The largest group of elements is
Metals.
NaHCO3
6
what state of matter has the least amount of energy
solid
is this an element or a compound
NaCl
Compound
flammability
chemical property
small numbers in a chemical formula
( the 2)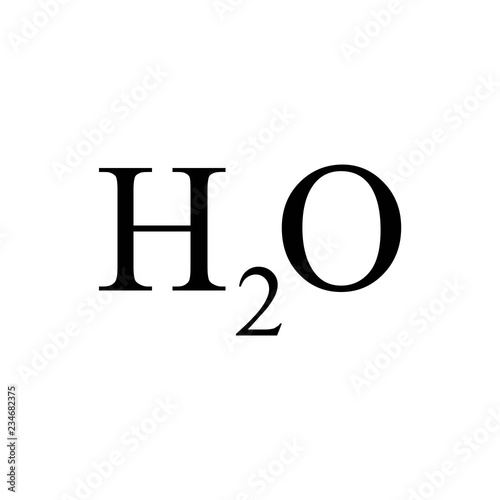
subscript
What do you add together to get the mass of an atom?
Protons and Neutrons
This group of elements is very poor conductors of electricity and heat.
Nonmetals.
C8H8O3
19
very high pressure can cause a liquid to turn to a ______________
a mixture or pure substance
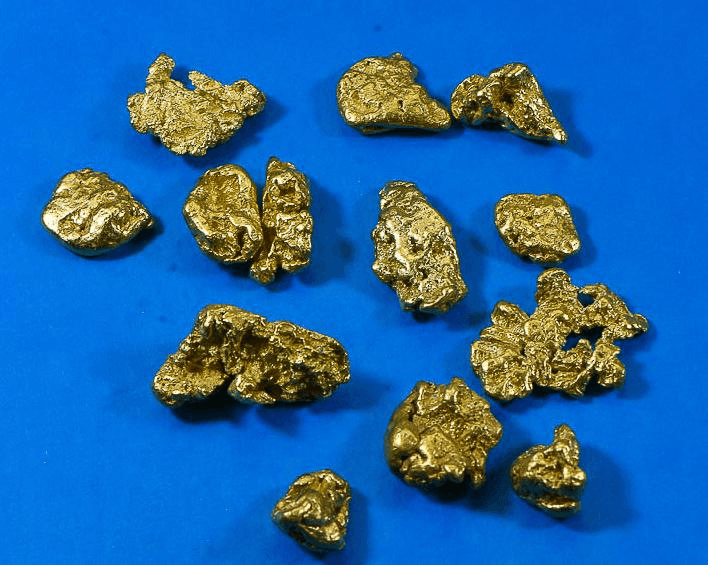
pure substance
heat is released
chemical change
the shortest month of the year
February
an atom is mostly made up of?
empty space.
this group of elements is ductile
Metals.
CaMg(CO3)2
10
when a substance turns straight from a solid to a gas
sublimation
element or compound
Mg
element
what type of change is this
physical change
how many stripes are there on the American flag
13
what is the name of the units we use to measure the mass of subatomic particles?
you must tell me the name, not just the letters.
Atomic
Mass
Unit
Sodium is a _________________
Metal, Metaloid, or Nonmetal

Metal
Al2(SO4)3
18
when a substance goes straight from a gas to a solid
Deposition.
which one is a mixture
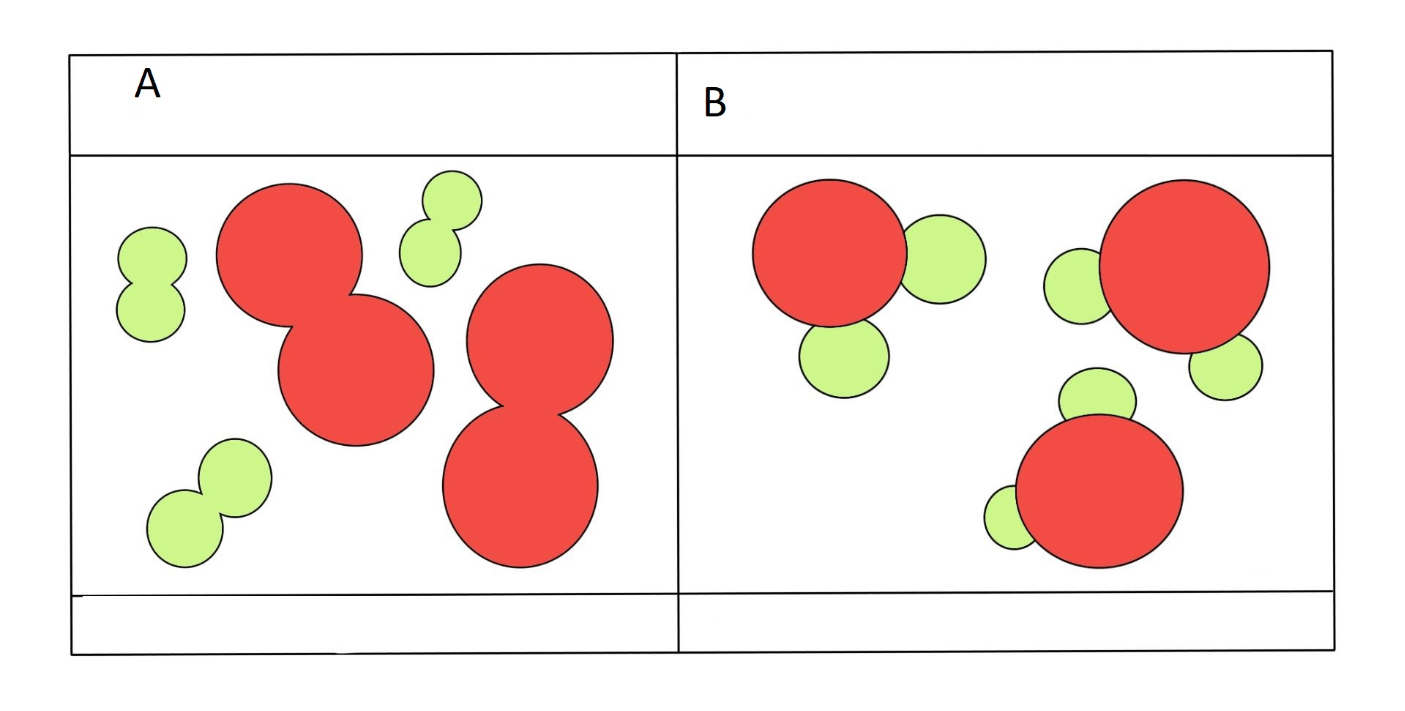
A
ice melting
physical change
a chemical reaction that absorbs heat
the electrons are located in the _____________
Electron Cloud
or
Electron shield
a small group of elements that is nonreactive.
Noble Gasses
C16 H18 Cl (N3 S)5
51
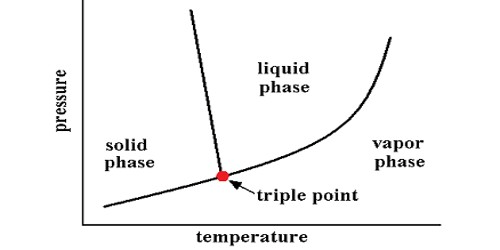
the point where a substance could be any 3 of the states of matter
Tripple point
which one is a compound 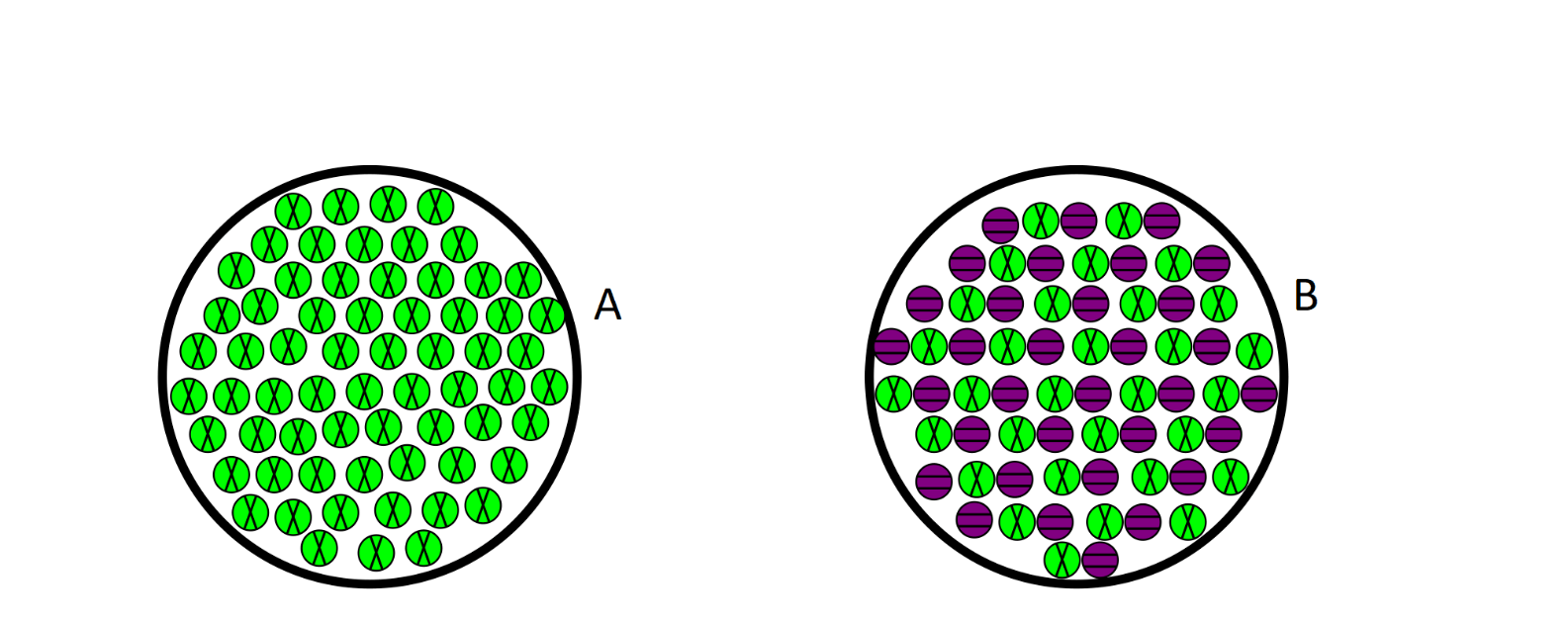
B
baking cookies

chemical
An element that explodes when it comes in contact with water.
Sodium, Na