How many electrons are involved in a double bond between carbon and oxygen?
4 electrons
What is the chemical formula for magnesium bromide?
MgBr2
What shape would a molecule have if it's center atom has 5 bonding electron pairs and no lone pairs?
Trigonal bipyramidal

Will the following reaction occur?
Al + CaCl2 -->
No. Aluminum is lower on the activity series than calcium.
What city are the 2024 Olympics being hosted in?
Paris, France
List the three primary IMFs in order from strongest to weakest.
Hydrogen Bonding > Dipole-Diploe > London Dispersion
What is the name of this compound?
S2F10
Disulfur decafluoride
Draw the Lewis Dot structure for ammonia (NH3)

Write the balanced equation with products for the following reaction:
Cl2 + CaI2 --> ______
Cl2 + CaI2 -->CaCl2 + I2
What was the first movie to win the Oscar for best animated film?
Shrek (2001)
In the compound HCN, what type of bond exists between carbon and nitrogen?
A triple covalent bond (non-polar)
What is the name of this compound:
CuOH
Is formaldehyde (CH2O) as a molecule polar or non-polar? Why?
Polar. C=O bond is polar and structure is asymmetric

What are the products of the following reaction? (also balance the equation)
C3H8 + O2 -->
C3H8 + 5O2 --> 3CO2 + 4H2O
Combustion
Which element has a larger 1st ionization energy, germanium or phosphorus?
Phosphorus
Between acetone (C3H6O) and propane (C3H8), which should have a higher boiling point and why?
Acetone will have a higher boiling point than propane, because acetone, as a polar molecule, has dipole-dipole IMFs, while propane, a non-polar molecule, just has dispersion IMFs. Stronger IMFs create a higher boiling point.
Explain the law of definite proportions.
A given chemical compound always contains its component elements in a fixed ratio and does not depend on its source and method of preparation
Draw the Lewis structure of XeF4, what is its molecular structure? Is the molecule polar?
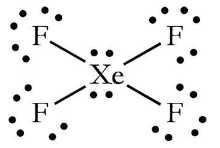
Square planar, non-polar
Balance the following equation:
_C3H8O + _O2 --> _CO2 + _H2O
2C3H8O + 9O2 --> 6CO2 + 8H2O
Describe the periodic trend for atomic radius and why it follows the pattern it does.
Across a period atomic radius decreases due to a larger positive nucleus pulling in electrons. Down a column atomic radius increases due to electrons occupying higher energy levels.
Explain on an atomic scale, the difference between non-polar covalent, polar covalent, and ionic bonds. What is happening to the electrons?
Points given by Dace's discretion
You have 15g each of two compounds containing just oxygen and sulfur. The first compound contains 7.5g of sulfur and the second compound contains 6g of sulfur. Show how this data illustrates the law of multiple proportions.
7.5g S -> 7.5g O 6g S -> 9gO
7.5gS/7.5=7.5gO/7.5 6gS/6=9gO/6
1g S = 1g O 1g S = 1.5g O
1.5gO/1gO=3/2
Draw the Lewis Dot structure for C6H6, including any resonance structures
(hint: the carbons want to be similar to each other bond-wise)

Write the balanced chemical equation for the reaction between potassium chromate & silver nitrate. Will the reaction occur? If so, what is the precipitate?
K2CrO4(aq) + 2AgNO3(aq) --> Ag2CrO4(s) + 2KNO3(aq)
The reaction will occur and silver chromate is the precipitate.
What is the term for the scientific study of bones?
Osteology. From the Greek ὀστέον (ostéon) meaning 'bones', and λόγος (logos) meaning 'study.