24.3 g/L * 3.020 L = ?
73.4 g
(3 sig figs, g/L*L cancels out to just g)
How many protons, neutrons, and electrons are in 2914Si+4 ?
Protons: 14
Neutrons: 15
Electrons: 10
What was the main aspect of the Thomson atomic model that Rutherford model improved upon?
The Rutherford model has the positive charge localized in the center of the atom (the nucleus). Thomson's model (the plum pudding model) had the positive charge evenly distributed throughout the whole atom.
(Points given on Dace's discretion)
How many cm are in 2.3km?
2.3x105cm (2.3km*1000m/1km*100cm/1m)
Which one(s) of the following are chemical changes:
Burning your toast Making Kool-aid with powder
A snowman melting Soda going flat over time
Burning your toast is the only chemical change
Complete the following nuclear equation:
22387Fr --> __ + 42He
22387Fr --> 21985At + 42He
Br-1 what what ending electron configuration?
4p6
183 times (2 weeks*7days/1week*24hrs/1day*60min/1hr*1FNAF/110min)
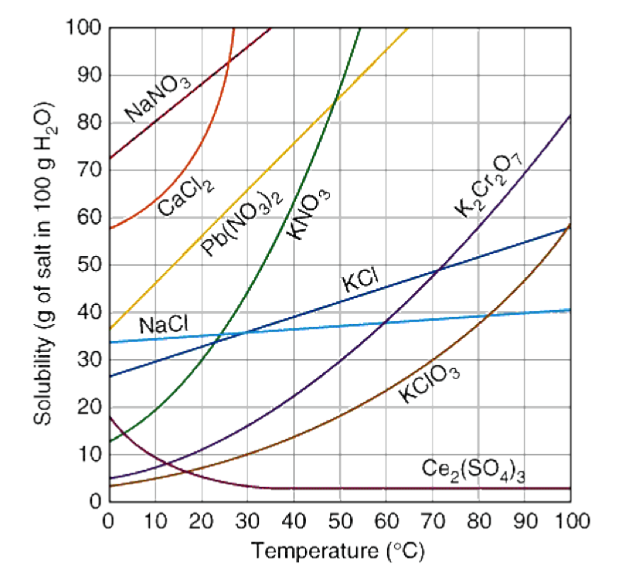
Based on the above solubility curve, 30g of potassium nitrate can be dissolved in water at or above what temperature?
20o C
Complete the following nuclear equation:
___ + 126C --> 24698Cf + 410n
23892U + 126C --> 24698Cf + 410n
Write the full electron configuration for Bismuth, plus the shortened notation.
Full: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p3
Shortened: [Xe]6s24f145d106p3
How many atoms of gold are in 10.1g of gold?
3.09x1022 atoms (10.1g*1mol/196.97g*6.022x10^23atoms/1mol)
Which of the following are NOT characteristic properties? (select all that apply)
Viscosity Temperature
Mass Reactivity
Mass & Temperature are not characteristic properties
You have 8g of Cf-252. 15.9 years ago you had 512g. What is Cf-252's half-life?
2.56 Years (512 -> 8 is 6 half lives, 15.9/6=2.56yrs)
A beam of light has 4.79x10-19J of energy. What is its frequency, wavelength, and color?
4.79x10-19J / 6.63x10-34Js= 7.22x1014 s-1 (frequency)
3.00x108m/s / 7.22x1014s-1 = 4.15x10-7m (wavelength)
415nm corresponds to a violet (purple) color
How many g of H2SO4 are required to be added to 20.mL of water to make a 0.45M solution?
.020L*0.45mol/1L=0.009mols required
0.009mol*98.08g/1mol= 0.88g Sulfuric acid
Draw an accurate, labeled graph of temperature vs time for gallium melting (Gallium's freezing point is 29.76oC)
Dace will award points & draw example
What is the final daughter nucleus if Plutonium-239 undergoes alpha decay twice, then beta decay once?
23994Pu --> 42He + 23592U
23592U --> 42He + 23190Th
23190Th --> 0-1e + 23191Pa
Why are the spectrum lines for a given element only specific colors? (use the terms energy level, fluorescence, quantized, & wavelength in your answer)
In the reaction between potassium & water, how many g of potassium are required to make 30.0g of hydrogen gas?
Equation: 2K + 2H2O --> 2KOH + H2
3.00gK*1molK/39.1g*1moH2/2molK*2.02gH2/1molH2=0.775g K