Lithium is a silver colored metal. It reacts chlorine gas to form lithium chloride. Which statement describes the properties of lithium chloride?
A. It will be a metal because lithium is a metal
B. Its properties will be different from lithium and chlorine because it will have some properties of each of the atoms that makes it up.
C. Its properties will be different from lithium and chlorine because properties depend on the type of atoms and how they are arranged.
C
Will a moon rock weigh more here on earth or on the moon?
The mass is constant but gravity is not. The rock will weigh more here on earth.
What is the atomic number of the element Cesium?
55
Salt crystals can be quite large and are even made into lamps and other decorative items. Which statement accurately describes the salt molecules?
A. They have no kinetic energy as they are in solid form.
B. They are not attracted to each other.
C. They have more kinetic energy than smaller salt crystals.
D. They have some kinetic energy but move around only in a limited location.
D
A cup of ice is pulled out of the freezer and placed on the counter top. Which is try about the number of particles in the cup?
A. There are fewer water particles after warming since some of the ice turn into air.
B. There are more water particles since liquid takes up more room than solid does.
C. There are more water particles since some of split apart to form water from ice.
D. The number of water particles are the same, including solid, liquid, and gas.
D
Which of these is not a model of NH3 (ammonia)?
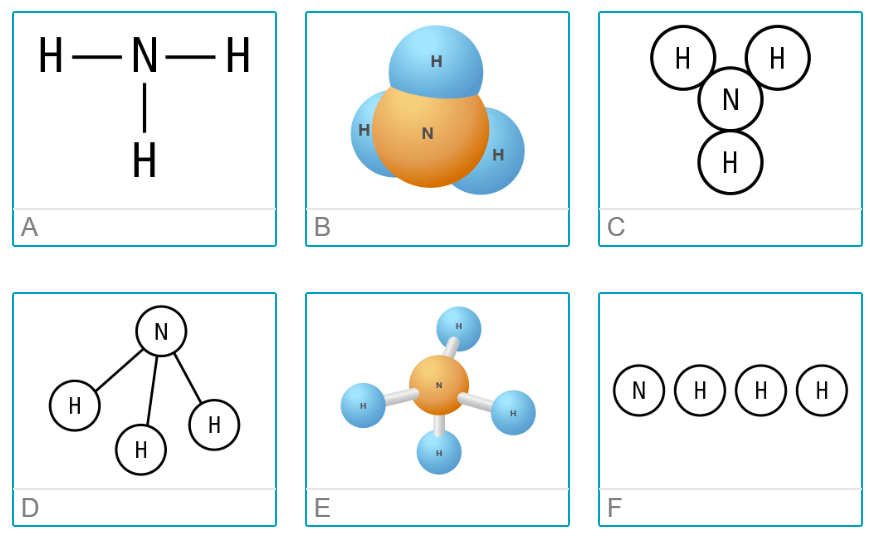
E and F
Two objects are the same size. Object A is twice as heavy as object B. Which object has higher density?
Object A is double the density of Object B.
What is the atomic mass of the element Pb?
Pb = lead so the atomic mass is 207.20
Which of the three states of matter, gas, liquid or solid, is has molecules that are packed together and but move past each other freely?
liquid
Dry ice is the common name for frozen CO2. Which picture shows the process of sublimation?
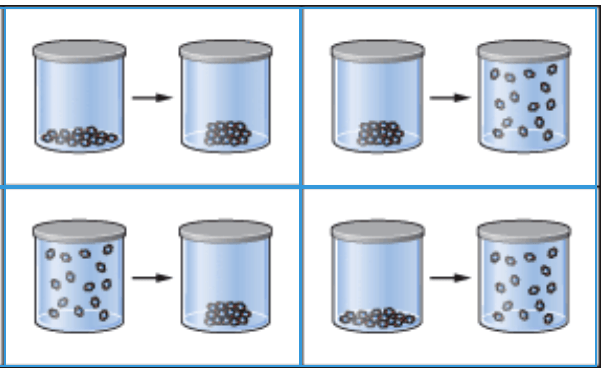
Top right hand picture.
Bill has iron flakes, salt and water. He makes a table to describe the different substances he has. Which boxes should he check for Iron (Fe)?
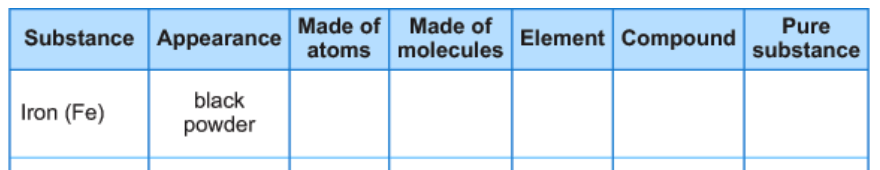
Made of atoms, element, and pure substance
Classify the following as matter or not matter:
carbon
fire
cloud
light
carbon and cloud are matter
fire and light are not matter
Which picture shows a crystal structure?
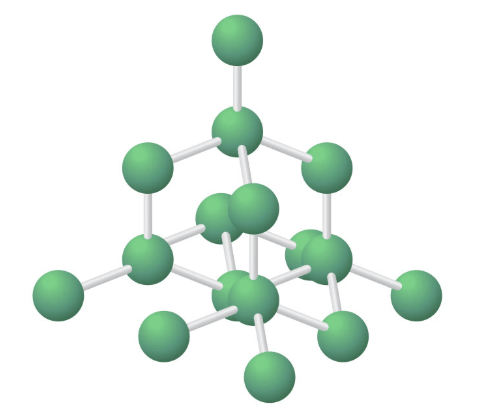
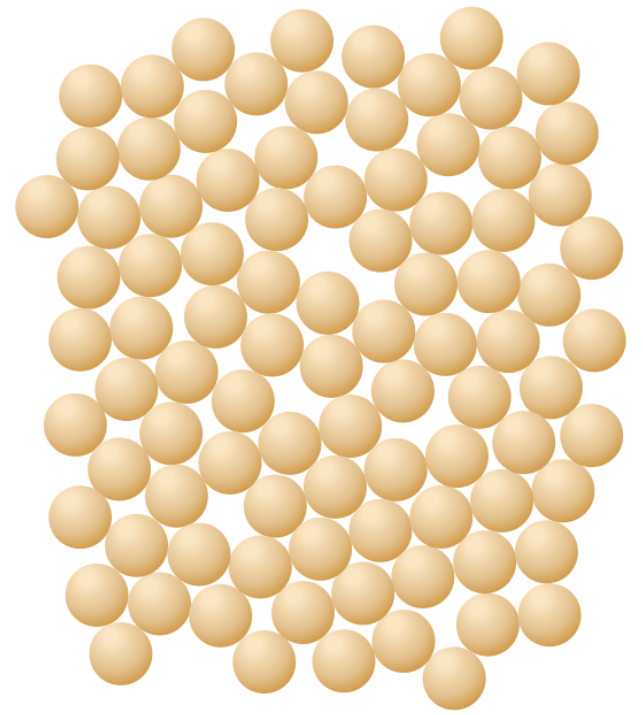
Top
Which state has the lowest density but highest volume?
gaseous state
Thermal energy is being added to a pure substance. Which section shows vaporization?
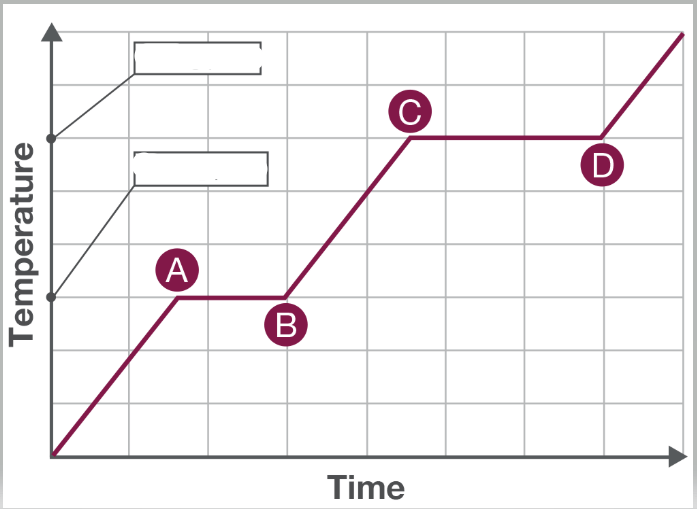
From C to D
Sort the following properties into either chemical or physical properties:
boiling point
reactivity to acid
magnetism
density
color
chemical properties: reactivity to acid, magnetism
Physical properties: density, boiling point and color
Joe wants to calculate the volume of his house key. How could he do it? Write down each step.
1. Fill a graduated cylinder with water
2. Measure the water
3. Add the key
4. Measure the water again and calculate the difference.
5. Volume of the key = difference of water volumes.
Cesium is frequently used for the older atomic clocks (newer ones use calcium). It is highly reactive. In what gas should the cesium be stored?
Any of the noble gasses, but argon is the most common.
Describe the volume, shape and kinetic energy of the solid, liquid and gas states.
solid: constant volume and shape, low kinetic energy
Liquid: constant volume, changeable shape, higher kinetic energy
Gas: changeable shape and volume, high kinetic energy
A balloon filled with nitrogen is placed in the freezer. Fill in the blanks below:
Thermal energy will ___________ in the balloon. For the particles in the balloon, the kinetic energy and motion will _____________. The nitrogen will change state only if the freezer is _________________
decrease
decrease
below the condensation point
Below is an image of saturated vs unsaturated fats. Saturated fats are solid at room temperature while unsaturated fats are not. Why?

Saturated fats form long straight chains of carbons. These pack together to form the solid. Unsaturated fats are bent and can't be packed closely together. This means they are liquid at room temperature.
Beth has two unlabeled balloons, one filled with hydrogen and the other with krypton gas. The density of hydrogen is 0.00523. The density of krypton is 3.74. How can she tell the which balloon has which gas?
Hydrogen is much less dense than air, while krypton is much more dense. Basically the hydrogen will float and the krypton will sink
How many electrons does an Dysprosium atom have? Hint: the number of electrons = number of protons in the atom
66 electrons for 66 protons in the Dysprosium atom
Put in order from lowest to highest total thermal energy:
A. Some attractive forces are present.
B. Attractive forces between particles have stopped.
C. Strong forces of attraction exist between particles.
C: Solid
A. Liquid
B. Gas
Cathy is using a pressure cooker to seal some jars. After a few minutes of boiling, Cathy releases the pressure in the pressure cooker so that she can remove the jars but does not change the temperature of the substances inside. How will this affect the particles in the water vapor inside the cooker?
A. They will collide with each other more often.
B. They will change states back into liquid water.
C. They will collide with the walls of the pressure cooker less often.
D. They will begin to collide with air particles rather than the liquid water particles.
C