Sig fig 500
1
Classify pizza
Heterogeneous mixture
Fluorine
Family Fluorine
Halogen
Element with highest electronegativity
Fluorine
Types of chemical bonds
Covalent, metallic, and ionic
Convert 1000 mL to L
1 L
Give an example of an element
Na, O, Cl, etc. (anything on the period table)
or O2, I2, O3, etc.
How many neutrons does O—14 have?
Mass - Atomic # = Neutrons
6 neutrons
Valence electrons He
2
Element with largest atomic radius
Fr
Shared electrons
covalent
sig fig 0.0010
2
Classify air (78% N2, 21% O2, 1% trace)
Homogeneous mixture
What is the charge for an atom with 20 protons, 20 neutrons, and 18 electrons?
+ 2
Valence electrons oxygen
6
Element in group 1 with highest ionization energy
H
Metal + non metal
ionic
Convert 100 m to km
0.1 km
Classify NaO
Compound
What is the mass number for an atoms with 20 protons, 22 neutrons, and 18 electrons?
42
20 Protons + 22 Neutrons
3 energy levels, 1 valence electron
Na
Most reactive metals
alkali metals
Low melting point
Covalent
What is the % error if the theoretical yield = 9 g
and the actual yield = 8.1 g
% error = [(theoretical value - actual value) / actual value] x 100
Sig fig!
10%
Classify O2
Element
How many electrons does Fluorine have?
9
family helium
Noble gases
least reactive family
noble gases
Sodium oxide
What is 28,000 in scientific notation?
2.8 x 104
Give an example of a chemical property
toxicity, flammability, reactivity
What is the atomic number for an atom with 17 protons, 18 neutrons, and 17 electrons?
17
family sodium
Alkali metals
most reactive non metals
halogens
Name: NF3
Nitrogen trifluoride
What is 5.6151 × 10-1 in decimal (standard) notation?
0.56151
Give an example of a physical property
Color, mass, density, volume, state of matter, texture, viscosity, etc.
APE
Atomic # = Protons = Electrons
Subatomic particles?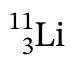
protons: 3
electrons: 3
neutrons: 8
element with electron configuration: 1s22s22p4
Oxygen
Chemical Formula: Copper (I) sulfide
Cu2S
Convert 0.02 g to cg
2g
cutting paper, ice cube melting, etc.
isotope
same # protons, different # neutrons
isotope name for a nucleus with 52 protons and 65 neutrons
tellurium-117
Name the family with + 2 oxidation number
Alkaline Earth Metals
Chemical Formula: trisulfur diiodide
S3I2