This is anything that has mass and takes up space
What is Matter
states that chemical and physical properties of elements are periodic functions of their atomic numbers
Periodic Law
this is the basic unit of a chemical element
Atom
when two or more atoms bond by sharing electrons
What is a Molecule
a chemical that cannot be broken down into another substance
what is an element
Matter (Atoms) can neither be created nor destroyed; atoms can only be rearranged
what is Law of Conservation of Mass
a solution that has an excess of H+ ions
What is an acid
when at atom has 8 electrons in the outermost shell
What is the Octet Rule
has definite volume and definite shape
what is a solid
study of the structure, properties, and reactions of carbon-containing compounds
What is Organic Chemistry
refers to the way a metal's surface reflects light
what is luster
This can be observed or measured without changing the identity of the substance
What are physical properties
This person developed the first periodic table in 1869
This has a positive charge
proton
Two or more atoms of the same element
What is an elemental molecule
an expression which states the number and type of atoms present in a molecule of a substance
a substance that increases the rate of a chemical reaction without changing chemically itself
What is a catalyst
a solution that has an excess of OH- ions
What is a base
transfer of electrons
what is an ionic bond
has definite volume, but NO definite shape
what is a liquid
Compose all life forms
Compounds of Carbon
Occur naturally in living organisms
what are biomolecules
refers to the way most metals can be stretched into wires
what is ductile
these are examples of what?
Size, Mass, Volume, Shape, color, texture, hardness, stiffness, solubility
What are Physical properties
_______ electrons are found in the outermost shell of an atom
Valence
this has a negative charge
electron
made of exactly 2 atoms
what is diatomic molecules
the small number after an elements symbol
what is a subscript
This involves Energy
what is a chemical reaction
Who is Svante Arrhenius
forms a solid at ordinary temperatures
What is an Ionic Bond
no definite volume or shape
what is a gas
do not contain carbon or hydrogen bonded together
What is inorganic compounds
tendency of a material to fracture or fail with a small amount of force
Burning the substance is used to do what?
what is to examine a substances chemical properties
This group is called the Halogens
17
atomic mass - atomic number tells you....
number of neutrons
two different elements bond to form molecules
what is a compound
the big number at the beginning of a chemical formula
what is a coefficient
releases excess energy in the form of heat, light, or even sound
what is an exothermic reaction
forms when atoms lose or gain electrons to have a fuller outer valence electron shell
what is an Ion
Sharing electrons
What is a covalent bond
two of more atoms, elements, or compounds close to each other, but not bonded together
what is a mixture
Building Blocks
what are monomers
Luster, Good Conductor of electricity and heat, high density, high melting point, ductile, malleable
what is a metal
This is an ingredient in Vinegar
What is Acetic Acid
Atoms with different numbers of neutrons
This is the Lewis Dot structure for.....
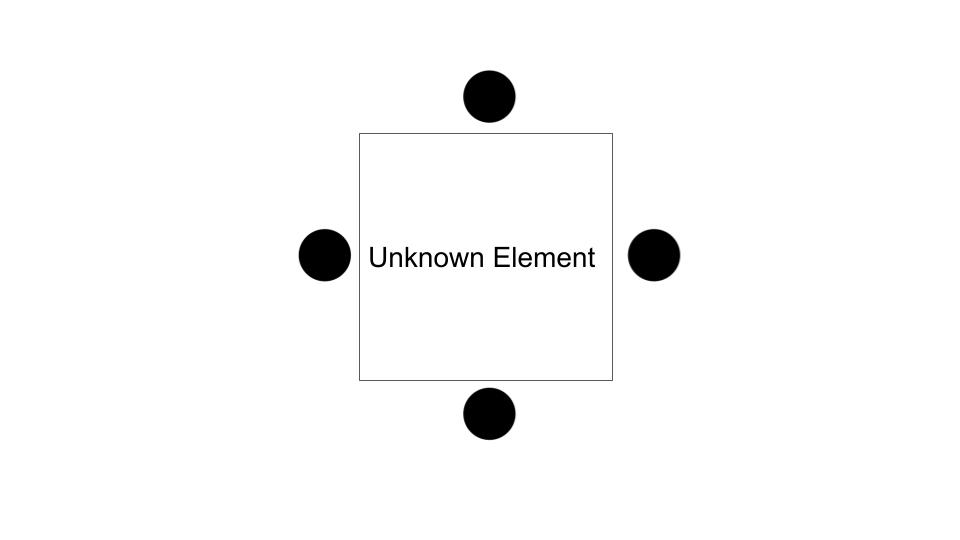
Carbon
When an atoms loses an electron
What is it will have a net positive charge
How many total atoms in the following molecule
2Al2(SO4)3
what is 34
requires more energy than they produce; will have a cold feeling
what is an endothermic reaction
what is a pH scale
Exists as gases, liquids, and solids
what is a covalent bond
Found in construction materials, ceramics, computer chips, rocks, and glass
What is Silicon
a large molecule made of monomer
what is a polymer
No luster, poor conductors, brittle, not ductile, not malleable, low density and melting point