Graph area where you can find the dependent variable.
What is y-axis?
Phase of matter where particles have the highest energy, are spaced out, and move at fast speeds.
What is a gas?
Exothermic or endothermic: Ice melting in the hand.
What is endothermic?
" __________ ____________ from where it's hot to where its not" (must be exact words)
What is "heat flows"?
How long you have to wash your eyes out in the eyewash station if you get chemicals in them.
What is 15 mins?
Convert 89.7 Celsius to Kelvin
What is 362.85 K?
Quantitative or qualitative: 20% of survey respondents bought ice cream today.
What is quantitative?
Order the following states of matter from highest to lowest KE: solids, liquids, gases, plasmas.
What is plasmas > gases > liquids > solids?
Find the change in temperature. Water has an initial temperature of 20C and a final temperature of 80C.
What is 60C?
Heat transfer on how the plates move in the earth.
What is convection currents?
Heat transferred via physical touch.
What is conduction?
The mass of an object is 7.3 grams, and it has a volume of 7.6 mL. Calculate the density of the object in g/mL.Write 2 decimal places.
What is 0.96 g/mL?
Accurate and/or precise: Five darts all strike near the center of the target, close to each other. Whoever threw the darts is...
What is both accurate and precise?
Letter(s) where you can find the three states of matter.
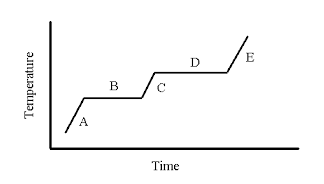
What are A, C, and E?
Give both the variable and units of the following:
- mass
- specific heat
- change in temperature
- heat
What are:
- mass = m (g)
- specific heat = c (J/gC)
- heat = Q (J)
- change in temperature =
DeltaT (C)
Best represents heat transfer by radiation.
- heat from a lamp
- ice melting in hand
- ocean currents cirulating
What is heat from a lamp?
Spreading of the ocean's floor due to convection currents under the surface.
What is seafloor spreading?
The specific heat of a 38.7 g sample of a substance that requires 259.9 J of energy in order to raise the temperature from 13.2C to 48.8C. Need 2 decimal places.
What is 0.19 J/gC?
The variable a scientist doesn't have control of during an experiment.
What is a dependent variable?
Float or sink: An object has a density of 1.8 g/mL and is in water, which has a density of 1 g/mL.
What is sink?
Letter where you can find ONLY liquid.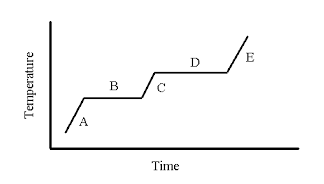
What is C?
Where the Earth get's its heat from to produce convection currents in the mantle.
What is the core?
True or false: The inner core is hotter than the outer core of the Earth.
What is true?
The variable that represents 23 degrees celsius based off the following question "A sample of water heats up from 0 degrees celsius to 23 degrees celsius"
What is...
delta T?
Solubility of Potassium Chloride (KCl) at 90C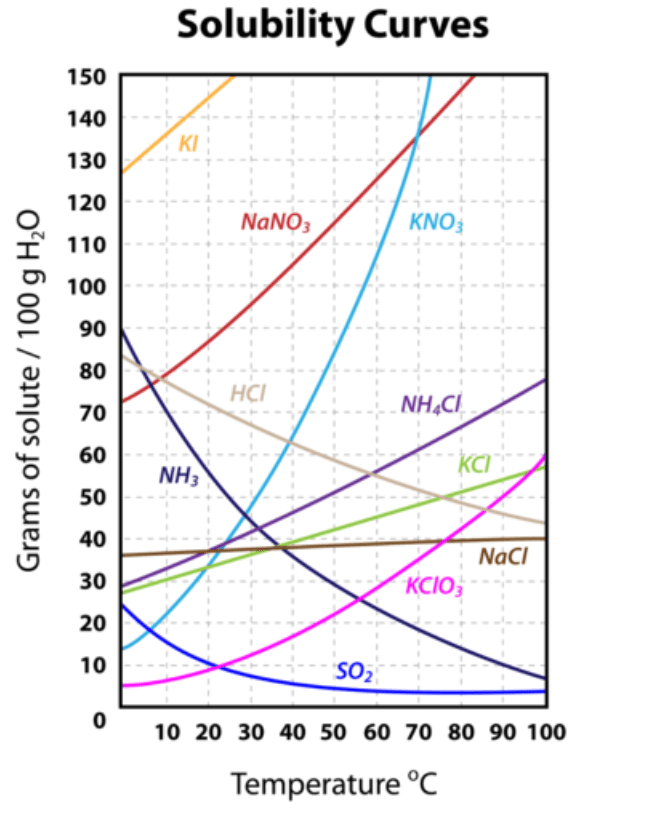
What is ~55 grams of solute?
Letters that have a greater IMF than KE.
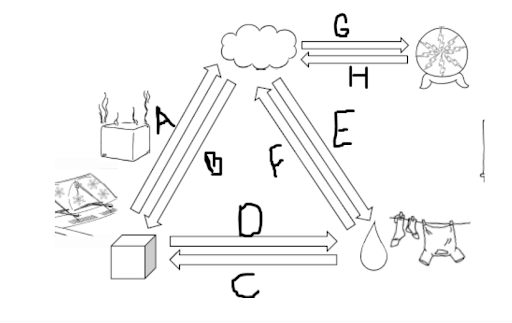
What are B, C, E, H?
Energy required to raise the temperature of 6.7 g of mercury metal from 5.9C to 71.0C. Specific heat of mercury is 0.138 J/gC. Need 2 decimal places.
What is 60.19 J?
A plate boundary thats movement consists of spreading plates apart from one another.
What is a divergent plate boundary?
The type of heat transfer that helps a hot air balloon rise.
What is convection?
If 40 degrees celsius is 104 degrees fahrenheit, would the temperature of 276.15 degrees kelvin be considered "hot"?
What is...
no?