Draw two different sized objects with the same density.
Should be different sizes with same spacing between particles.
Define property of matter
attribute, quality or characteristic of matter.
Name a covalent compound.
Answers will vary. Has to be accurate
Why do atoms bond?
1mol
The relationship between density and volume.
As the volume increases, density decreases. Or as volume decreases, density increases.
List 3 chemical vs physical properties
Answers will vary
Name an ionic compound.
Has to be accurate
What bond shares electrons?
covalent
Whats the molar mas of ammonium nitrate?
80.022g/mol
What is the density of an object with 6.72mL and 7.21g?
1.0729g/mL or 1.07g/mL (g/cm3 is also legal)
Draw a model that includes a pure substance, mixture, compound, and an element in two drawings.

You have 7 carbons and 10 fluorines. What is the formula and the name of the compound?
C7F10
heptacarbon decafluoride
What is the noble AND valence electron configuration of Iron?
[Ar] 4s23d6
4s2
You have Element Gradium, which has three isotopes. Gr-100, Gr-90, and Gr-80 which are 10%, 80%, and 10% respectively. Whats the average atomic mass?
90amu
What is the mass of an object if you dropped it in a 100mL of liquid changes the volume to a final volume of 112mL. Also who's density is 2.34g/mL.
28.08g
List 3 intensive properties of matter. (doesn't change with amount)
color, density, ductility, specific heat, melting point, boiling point, flammability, etc.
AgNO3
Draw a lewis dot model of C6H10
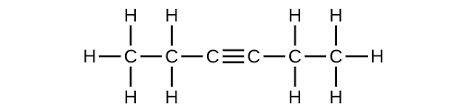
How many grams is 5.7mols of sodium phosphate?
934.8g
What is the density of an prism object if the mass is 3.5g and the sides are 2.2cm x 2.4cm x 2.5cm?
0.2652g/cm3 or 0.27g/cm3
What is the difference between a chemical and physical change?
Physical doesn't break bonds or chemical structure and a chemical change does.
Whats the name of PbSO4?
lead (II) sulfate
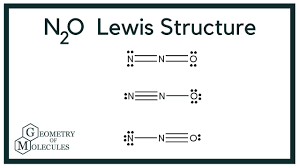
Lewis Dot of dinitrogen monoxide
How many molecules is 0.92g of trinitrogen tetrabromide?
1.53x1021molecules